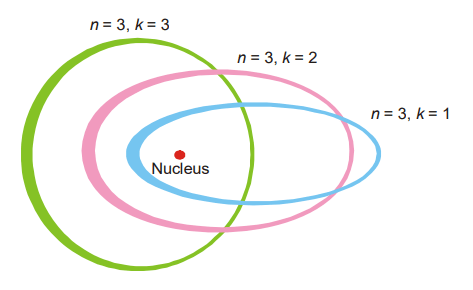
What is the Zeeman effect?
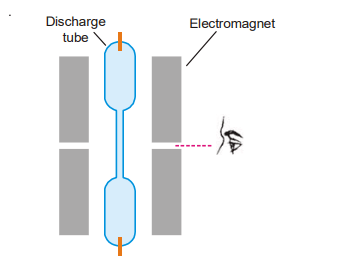
When a source producing lines is put in a high magnetic field, spectral lines are broken up into components, as found by a scientist named Zeeman in 1896. The effect is known as the Zeeman effect after its discoverer. The Zeeman effect was observed using the apparatus depicted in the diagram below. It comprises electromagnets … Read more