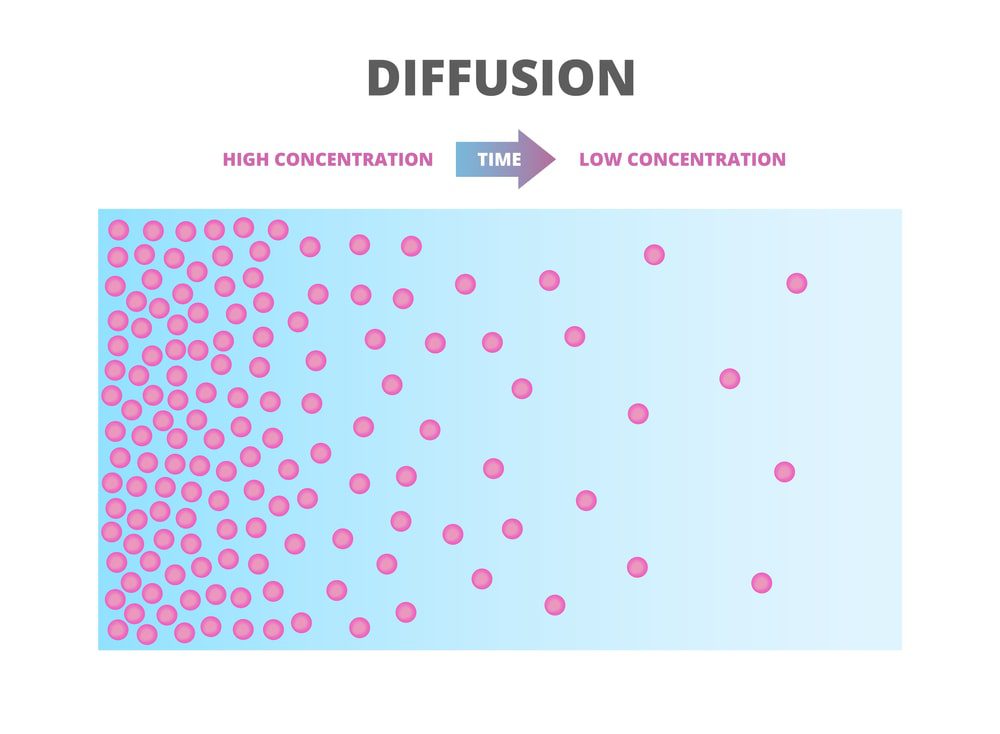
Matter moves by diffusion along energy gradients from areas of high concentration to areas of lower concentration. The rate of diffusion depends on temperature, the size of the particles, and the size of the concentration gradient. In biology, the selectively permeable cell membrane creates two special forms of diffusion, namely, osmosis for the diffusion of water and dialysis for the diffusion of solutes.
Diffusion is one principal method of movement of substances within cells, as well as for essential small molecules to cross the cell membrane. Cell membranes act as barriers to most, but not all, molecules. A cell membrane that could allow some materials to pass while preventing the movement of other molecules is a major step in the development of the cell. The cell membrane functions as a semi-permeable barrier, allowing very few molecules to cross it while holding the majority of chemicals inside the cell. Cell membranes separate the inner cellular environment from the outer cellular (or external) environment. Most of the molecules move from higher to lower concentrations, although there will be some molecules that move from low to high. The overall movement is thus from high to low concentration. If there is no energy input into the system, the molecules reach a state of equilibrium and get uniformly distributed throughout the system.
A cell membrane is composed of phospholipids and proteins. Absorption of drugs across the stomach lining/mucosa and the blood/brain barrier are two representative examples of transport phenomenon. Skin is another great example of a membrane for the entry of drugs. The transport of drug molecules through a non-porous membrane occurs by diffusion. Transport through porous cell membranes occurs by diffusion and convection.
The rate of diffusion is expressed by the equation dM/dt=DSK(C1 − C2)/h
Where M is the amount of drug dissolved, t is time, D is the diffusion coefficient of the drug, S is the surface area of the membrane, K is the oil/water partition coefficient, h is the thickness of the liquid film, C1 is the concentration of drug at donor side of the membrane, C2 is the concentration of drug at receptor side, and C1-C2 is the concentration gradient. However, C1 and C2 are not measured since these values varies within the membrane.
Typically, the gradient is measured as Cd − Cr, representing the partition at each phase, namely Ko/w = C1/Cd and Ko/w = C2/Cr. The rate of drug transport into a diffusional system is predominantly dependent upon the magnitude of the concentration gradient, considering the other parameters are constant.
Water, carbon dioxide, and oxygen are among the few simple molecules that can cross the cell membrane by diffusion (or a type of diffusion known as osmosis). Gas exchange in the lungs operates through the diffusion process. All cells, because of cellular metabolic processes, produce carbon dioxide. Since the source is inside the cell, the concentration gradient is constantly being replenished/re-elevated; leading to a net flow of CO2 out of the cell. Metabolic processes in animals and plants usually require oxygen, which is in lower concentration inside the cell, and have the net flow of oxygen into the cell through diffusion.
Also read: What is a solubility