The solubility of gas in liquids is the concentration of dissolved gas in the liquid when it is in equilibrium with the pure gas above the solution. Examples of gas in a liquid include effervescent preparations containing dissolved carbon dioxide, ammonia water, and hydrochloride gas. Aerosol products containing nitrogen or carbon dioxide as propellant are also considered to be solutions of gases in liquids.
Factors Affecting the Solubility of Gas in Liquids:
The solubility of gas in liquids depends on pressure, temperature, salt present, chemical reaction, and micellar solubilization.
Pressure:
Liquids and solids exhibit practically no change in solubility with changes in pressure. When considering the solubility of gases in liquids, the pressure of the gas in contact with the liquid is important. At higher gas pressure, more gas is dissolved in liquids (Fig. 1.6). For example, the soda bottle is packed at high pressure with carbon dioxide before sealing. When the cap of the bottle is opened, the pressure above the liquid is reduced to 1 atm, and the soda fizzes. This fizzing is just carbon dioxide that was dissolved in soda getting released. Therefore, if the pressure is lower, less carbon dioxide is soluble.
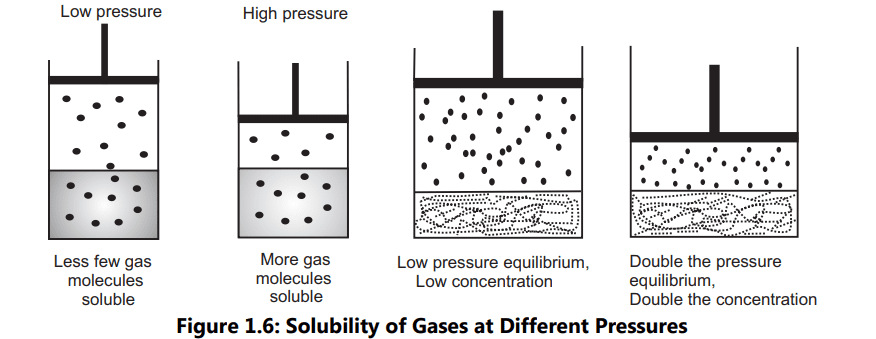
The effect of pressure on the solubility of gas is given Henry’s law states that in a dilute solution, the mass of gas that dissolves in each volume of liquid solvent at a constant temperature is directly proportional to the partial pressure of gas. Mathematically, it is expressed as SG = KH PG… (1.3)
Where SG is the solubility of gas, expressed as mol/L; KH is the Henry law constant which is different for each solute-solvent system and Pg is the partial pressure of the gas in mmHg. The amount of undissolved gas above the solution is obtained by subtracting the vapor pressure of the pure liquid from the total pressure of the solution.
Also read: Factors influencing solubility of drugs