Atomic spectrum of hydrogen
The emission line spectrum of hydrogen can be obtained by passing an electric discharge through the gas contained in a discharge tube at low pressure. The light radiation emitted is then examined with the help of a spectroscope. The bright lines recorded on the photographic plate constitute the atomic spectrum of hydrogen showing in the figure below.
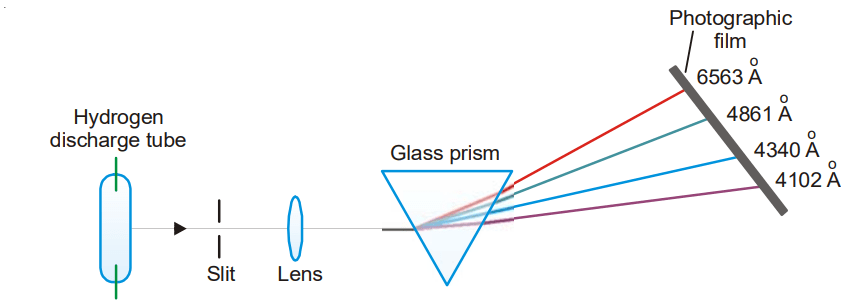
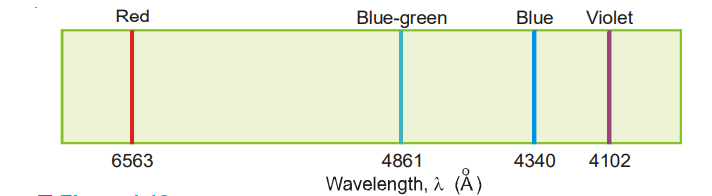
In 1884 J.J. Balmer observed that there were four prominent colored lines in the visible hydrogen
spectrum :
(1) a red line with a wavelength of 6563 Å.
(2) a blue-green line with a wavelength of 4861 Å.
(3) a blue line with a wavelength 4340 Å.
(4) a violet line with a wavelength 4102 Å.
The Balmer Series refers to a set of four lines in the visible spectrum of hydrogen. Balmer was able to experimentally give an equation that linked the wavelengths (λ) of the observed lines by carefully analyzing the wavelengths of the observed lines. The Balmer equation is 1/ λ = R(1/22 -1/n2 )
Here R is a constant called the Rydberg Constant which has the value 109, 677 cm– 1 and n = 3, 4,
5, 6, etc. That is, if we substitute the values of 3, 4, 5, and 6 for n, we get, respectively, the wavelength
of the four lines of the hydrogen spectrum.
In addition to Balmer Series, four other spectral series were discovered in the infrared and
ultraviolet regions of the hydrogen spectrum. These bear the names of the discoverers. Thus in all,
we have Five Spectral Series in the atomic spectrum of hydrogen :
| Name | Region where located |
| (1) Lyman Series | Ultraviolet |
| (2) Balmer Series | Visible |
| (3) Paschen Series | Infrared |
| (4) Brackett Series | Infrared |
| (5) Pfund Series | Infrared |
Balmer equation had no theoretical basis at all. Nobody had any idea how it worked so accurately in finding the wavelengths of the spectral lines of the hydrogen atoms. However, in 1913 Bohr put forward his theory which immediately explained the observed hydrogen atom spectrum. Before we can understand Bohr’s theory of atomic structure, it is necessary to acquaint ourselves with the quantum theory of energy.
Make sure check our amazing article on: Quantum theory and Bohr atom model