Table of Contents
Bohr atom model
Rutherford’s model laid the foundation of the model picture of the atom. The Rutherford model, on the other hand, provided no information on the position of the electrons or how they were organized around the nucleus. Rutherford saw that electrons were circling the nucleus. However, according to classical physics, an electron traveling in a force field similar to that of a nucleus would emit radiations and eventually collapse into the nucleus. As a result, the Rutherford model was unable to explain why electrons were unable to do so. Neils Bohr, a brilliant Danish Physicist, pointed out that the old laws of physics just did not work in the submicroscopic world of the atom. He closely studied in his Bohr atom model, the behavior of electrons, radiations, and atomic spectra.
In 1913 Bohr proposed a new model of the atom based on the modern Quantum theory of energy. With his theoretical model, he was able to explain why an orbiting electron did not collapse into the nucleus and how the atomic spectra were caused by the radiations emitted when electrons moved from one orbit to the other. Therefore to understand the Bohr theory of the atomic structure, it is first necessary to acquaint ourselves with the nature of electromagnetic radiations and the atomic spectra as also the Quantum theory of energy.
Electromagnetic Radiations
Electromagnetic radiation can transport energy over space. Radio waves, visible light, infrared light, ultraviolet light, X-rays, and -radiations are examples of radiant energy.
Electromagnetic radiations are so named because they consist of waves that have electrical and magnetic properties. An object sends out energy waves when its particles move up and down or vibrate continuously. Such a vibrating particle causes an intermittent disturbance that constitutes a wave. A wave conveys energy from the vibrating object to a distant place. The wave travels at the right angle to the vibratory motion of the object.
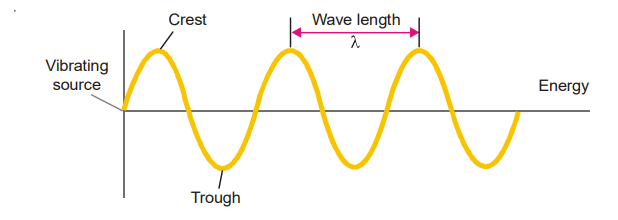
Waves similar to electromagnetic waves are caused when a stone is thrown in a pond of water.
The stone makes the water molecules vibrate up and down and transmit their energy as waves on the water
surface. These waves are seen traveling to the bank of the pond.
As in the case of water or sound waves, a wave can be created by the actual movement of particles in the medium. Electromagnetic waves, on the other hand, are created by the periodic motion of charged particles. As a result of the vibratory motion of electrons, a wave train of oscillating electric field and another oscillating magnetic field would be produced. These electromagnetic waves travel at the speed or velocity of light through empty space.
Characteristics of Waves
A series of waves produced by a vibrating object can be represented by a wavy curve of the type
shown in the Figure above. The tops of the curve are called crests and the bottoms troughs. Waves are
characterized by the following properties.
Wavelength
The wavelength is defined as the distance between two successive crests or troughs of a wave.
Wavelength is denoted by the Greek letter λ (lambda). It is expressed in centimeters or meters or in angstrom units. One angstrom, Å, is equal to 10-8 cm.
It is also expressed in nanometers (1nm = 10-9 m). That is, 1 Å = 10-8 cm = 10-10 m or 1 cm = 108 Å and 1 m = 1010 Å 1 nm = 10-9 m
Frequency
The frequency is the number of waves that pass a given point in one second. Frequency is denoted by the letter ν (nu) and is expressed in hertz (Hz). It is noteworthy that a wave of high frequency (b) has a shorter wavelength, while a wave of low frequency (a) has a longer wavelength.
Speed
The speed (or velocity) of a wave is the distance through which a particular wave travels in one second.
The symbol for speed is c, and it is measured in centimeters per second. If a wave’s speed is c cm/sec, it implies the wave’s distance traveled in one second is c cm. The equation relates speed to frequency and wavelength.
c = νλ
or Speed = Frequency × Wavelength
The wavelengths and frequency of various kinds of electromagnetic radiation vary. Because all kinds of radiation have the same speed or velocity, This velocity has been calculated experimentally and is 3 × 1010 cm/sec = 186,000 miles per second, which is the same as the speed of light.
Wave Number
Another quantity used to characterize radiation is the wavenumber. This is reciprocal of the wavelength and is given the symbol `v (nu bar). That is, `v = 1/λ The wavenumber is the number of wavelengths per unit of length covered. Its units are cm-1 or m-1.