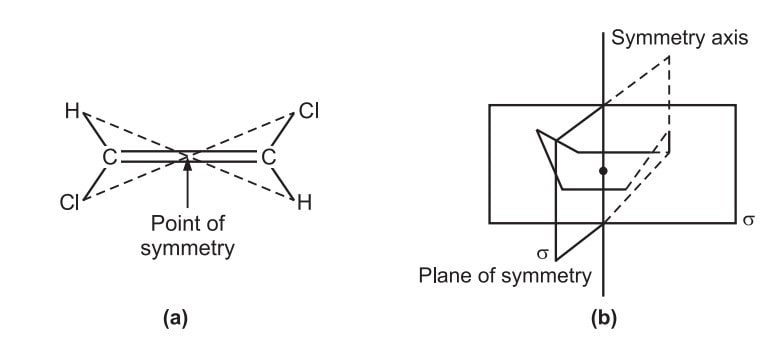
Conditions for optical activity
Conditions for optical activity : The two aryl groups’ planes must not be plane. Bulky groups are positioned in the ortho locations to achieve it. (ii) In most cases, the enantiomers can be resolved. (iii) Ortho substituents increase the restricted rotation by their steric repulsion. (iv) Mono ortho-substituted biaryl compounds do not show atropisomerism at … Read more