Table of Contents
Asymmetric synthesis (partial and absolute)
Asymmetric synthesis: De novo conflation of a chiral substance from an achiral precursor similar that one enantiomer predominates over the other is called asymmetric conflation. For responses where motes formerly contain a chiral element and conflation introduces a new chiral element, conflation is appertained to as’ stereoselective or enantioselective’ conflation or diastereoselective conflation.
Decarboxylation of ethyl methyl malonic acid to give α methylbutyric acid is one of the first recorded asymmetric synthesis.
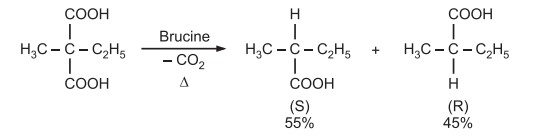
- Generally chiral reagents are used to carry out the reaction, if they are not available, chirality is acquired upon chelation, solvation etc.
- Reactants are adsorbed onto chiral surfaces or within chiral crystals.
- Chiral adjuvant or chiral auxiliary is temporarily attached to the achiral substrate which is cleaved after the synthesis by hydrolysis to recycle the adjuvant.
- When a new stereogenic center is created in achiral molecule we get a racemic mixture while in diastereoselective synthesis, formation of any one of the desired diastereomer is preferred over the other.
Typical asymmetric syntheses include
- Asymmetric hydrogenation
- Asymmetric epoxidation
- Asymmetric dihydroxylation
In contrast to “absolute” asymmetric synthesis, which used physical reagents like circularly polarised light, partial asymmetric synthesis refers to the preparation of optically active compounds from achiral compounds through the intermediate use of optically active compounds as reagent without the need for resolution.
1. Asymmetric Hydrogenation (Reduction)
It is used for asymmetric synthesis of analgesic drug Naproxen.
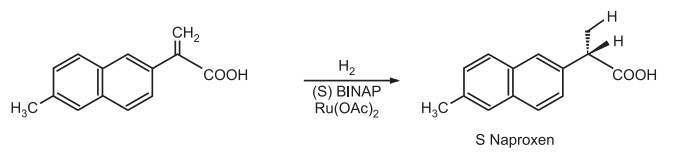
For double bonds bearing amino group, better catalysts are based on rhodium.
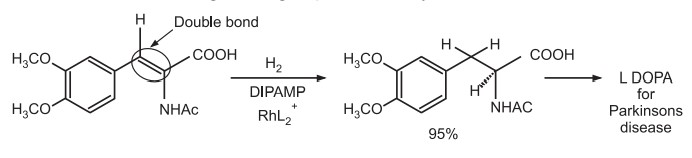
The catalyst is a cationic complex of rhodium with another diphosphine DI PAMP.
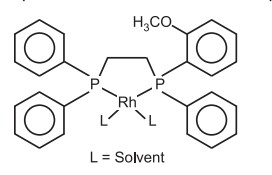
Important application of asymmetric hydrogenation is in synthesis of L menthol from (R) citronellal.
2. Asymmetric epoxidation
Oxidation of alkenes by asymmetric epoxidation is one of the popular sharpless reaction.
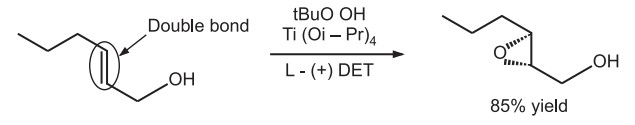
Catalyst is a transition metal, Titanium tetraisopropoxide with tertiary butyl hydroperoxide. The ligand is diethyl tartarate which is chiral and imparts selectivity to the reaction.
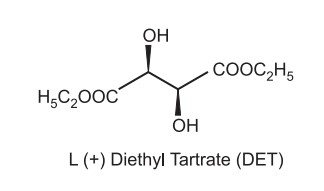
Only allylic alcohols can be oxidised using this type of metal catalyst. Two titanium atoms connected by two tartrate ligands create the first active compound. Each titanium atom is co-oridinated to one of the tartrate ligand’s carbonyl groups and retains two of its isopropoxide ligands. One of the remaining isopropoxide ligands and one of the tartrate carbonyl groups are both moved by the addition of the oxidising agent tBuOOH. As another isopropoxide ligand is displaced by the titanium, further allylic alcohol is coordinated.
Because of the shape of the complex of the reactive oxygen atom of the bound hydroperoixde has to be delivered to the lower face of alkene and epoxide is formed in high enantiomeric excess.
Epoxides easily react with many nucleophiles to give 1,2, disubstituted products and thus used in synthesis of drugs e.g. Propranolol- used as β blocker.
3. Asymmetric dihydroxylation:
Dihydroxylation of alkenes by osmium tetroxide in catalytic amount is carried out.
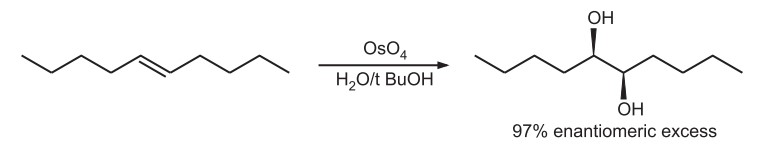
- Osmium (VIII) act as oxidizing agent and K3Fe (CN)6 is commonly used to reoxidize the osmium after each catalytic reaction.
- To increase rate of reaction K2CO3 and methanesulfonamide are added.
- Chiral ligands are usually alkaloids dihydroquinidine and dihydroquinine based which must be attached to aromatic ring e.g. Phthalazine.
- Trans alkenes dihydroxylates more selectively than other alkenes because of alignement of ligand and catalyst.
- Reaction has been successfully used for synthesis of antibiotic chloramphenicol in few steps.
Energy Profile diagrams for asymmetric synthesis
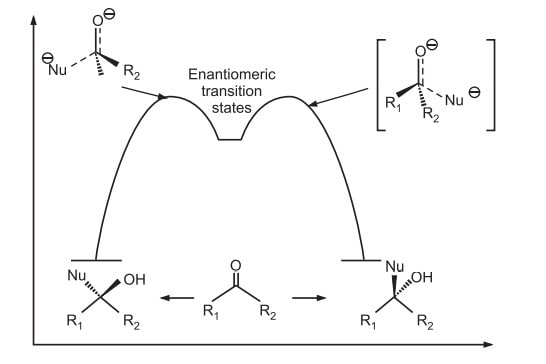
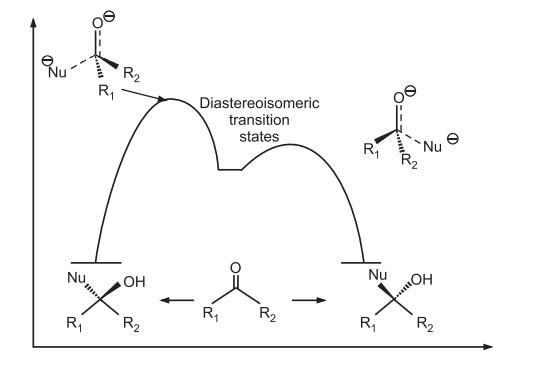
Also read: Reactions of chiral molecules