Reactions of chiral molecules : Chiral molecules react with the reagents in a variety of ways, and accordingly, reactions are classified as follows:
- Reactions where bonds with the chiral center are not broken.
- Reactions leading to generation of chiral center.
- Reactions of chiral compounds with optically active reagents.
- Reactions where bonds with the chiral center are broken.
Table of Contents
Reactions of chiral molecules
1. Reactions where bonds with the chiral center are not broken.
These reactions can be used to relate the configuration of one compound to that of another. Configuration is retained when the reaction does not involve the breaking of a bond to a chiral center.
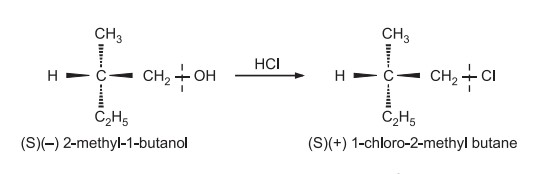
Here the bond to the chiral center is not broken ‘S’ configuration is retained, because ‘–CH2–Cl’ occupies the same relative position as that was occupied by –CH2OH in the reactant. This retention of configuration can be utilized to determine the configurational relationship between two optically active compounds by converting them into each other by reactions that do not involve the breaking of a bond to a chiral center. Only relative configuration can be assigned than absolute configuration.
Such reactions are used to get specific rotations of optically pure compounds. e.g. 2-methyl-1-butanol from fusel oil has a specific rotation of –5.90° and is optically pure. Upon treatment with hydrogen chloride, 1-chloro 2-methyl butane has a specific rotation of +1.67°. So if a sample has rotation equal to this value, the compound is said to be pure. If rotation is about + 0.8º, the compound is said to be only 50% optically pure.
2. Reactions leading to generation of chiral center:
Generation of the first chiral center in a compound usually yields equal amounts of enantiomers (Racemic mixture) but reactions that form the second/new chiral center yield unequal amounts of diastereomers depending on the side of attack.
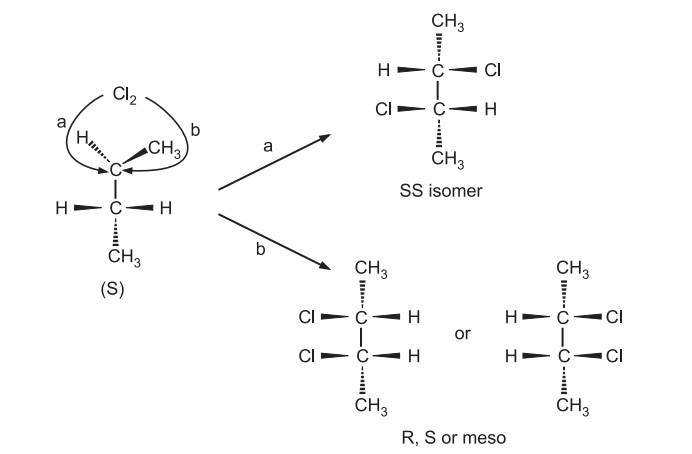
Retention of configuration(s) occurs as there is no bond breaking to the chiral center. For the new chiral center, depending on side of attack from the same or opposite side, diastereomers are formed but in unequal amounts. This is because the intermediate 3-chloro-2-butyl radical contains a chiral center and it lacks symmetry. So two faces of the molecule for attack are not equal to each other. Here S isomer would yield the SS and meso compound in the ratio of 29: 71.
In some reactions, both configurations may not be actually generated but probability exists. Similarly, R isomer would yield RR and meso compound in the ratio of 29: 71. If the reactant is optically inactive, it yields optically inactive products.
3. Reactions of chiral compounds with optically active reagents
In the resolution or separation of a racemic mixture/modification into separate enantiomers, such reactions are routinely used. Enantiomers are not separated by fractional distillation or crystallization because they have similar physical properties (except for optical rotation).
Optimally active reagents are used to create pure enantiomers following racemic modification. This type of optically active reagent can be easily obtained from natural sources or made from readily accessible chemicals.
Common reactions are reactions of organic acids and bases to form salts.
e.g. Reaction of racemic acid (+) HA with alkaloid reagent (–) B.
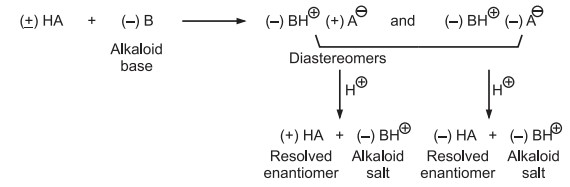
Different physical properties distinguish formed diastereomers, which can be easily separated via fractional distillation or crystallisation. Mineral acid can also be used to recover resolved enantiomers from the solution.
(–) brucine, (–) quinine, (–) strychnine, and other alkaloid bases are routinely utilised.
Acid reagents, such as (–) malic acid, can also be used to separate racemic bases. Additional than acids and bases, other compounds can be resolved. Because alcohols are weakly ionised and do not have a significant acidic or basic component, connecting them to an acidic handle that can be removed later aids in their resolution.
4. Reactions where bonds with chiral center are broken
Stereochemistry of such reactions depend on the mechanism of the reaction. Hence, stereochemistry can be helpful to give evidence of a particular mechanism.
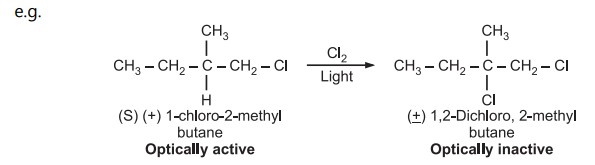
As the product is optically inactive and a racemic mixture, it implies second chlorine can be attached from either face of the intermediate, which can be a free alkyl radical with loss of chirality
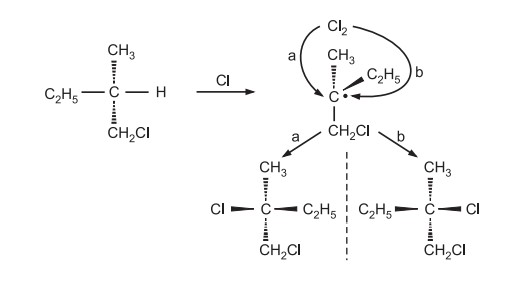
If there is a simultaneous attack of chlorine while the displacement of hydrogen, only the product from the backside attack of chlorine would have been obtained instead of the optically inactive product, so the mechanism involving free alkyl radicals is correct.
– A reaction is stereospecific when reactants exist as steroisomers and each isomeric reactant gives a different stereoisomeric product.
– A reaction is stethereoselective when reactant irrespective of any stereoisomerism produces predominantly or exclusively one stereoisomeric form of the product than other possible forms.
Nomenclature of optical isomers