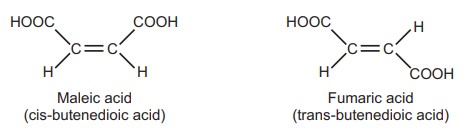
Geometrical isomerism: The molecular formulas of fumaric acid (m.p. 287 °C) and maleic acid (m.p. 130 °C) are identical, but the configuration of functional groups surrounding the double bond varies. They differ in terms of their physical and, to some degree, chemical characteristics. This type of isomerism is known as geometrical isomerism.
Nomenclature of geometrical isomers
Cis-trans Nomenclature: The flexibility of rotation about a double bond is constrained when there is a carbon-carbon double bond present. When similar atoms or groups are present on the same side, they are said to be cis (Latin for “same side”), and when they are present on the opposite side, they are said to be trans (Latin for “across”). When a double bond causes isomerism in a non-cyclic, open-chain chemical, it is referred to as diastereoisomerism; nevertheless, when a double bond is absent from a cyclic skeleton, it is referred to as diastereoisomerism.
E/Z System of Nomenclature: The simple convention of denoting the geometrical isomers by cis/trans is not possible when there are more than two different substituents on a double bond. Hence a new system of nomenclature known as the E/Z notation method is to be adopted.
Except for very simple alkenes, the nomenclature for alkene officially uses E/Z notation. In cyclic alkanes, cis and trans terminology is retained. The E/Z notation is not used in cyclic alkanes.
The group of highest priority on double bonded carbon atoms is first chosen according to the Cahn-Ingold-Prelog (CIP) priority sequence rule.
For example, in the following structure,
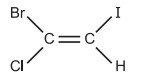
Here, Br > Cl and I > H are the order of the functional groups linked to the two double-bonded carbon atoms. It indicates that the functional groups with the highest importance are on the same side of the double bond (i.e., cis-isomer). As a result, it is called Z-form (from the German word, Zussamen meaning together). E-form is the term used when the highest priority functional groups are on the opposite sides of the double bond (i.e., trans-isomer) (from the German word, Entagagen meaning opposite). As a result, E denotes the opposite side and Z, the same side. With very few exceptions, cis isomer is typically referred to as Z-form and trans isomer as E-form.
CIP Rules for determining priorities:
(a) The atom which has the highest atomic number is given higher priority, in the case of simple structure, and
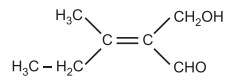
(b) The priority depends on the atomic weights of the other atoms in the group in the most complex molecule, where a group of atoms makes up the functional groups connected to the double bond’s carbons. For instance, in the provided compounds, CH3CH2 – > CH3 and CHO > CH2OH are the order of importance. Due to the double bond between carbon and oxygen in CHO, oxygen is counted twice. The suggested structure, therefore, has Z-form. Treat an atom as though it were singly bonded to three of those atoms when it is triple bonded to another atom.
(c) For molecules with multiple double bonds, it is necessary to indicate the alkene location for each E or Z symbol. For example, the prefix (2E, 4E, 6Z, 8E) used in the IUPAC name of alitretinoin indicates that the alkenes starting at position 2,4,8 are E while the alkene at position 6 is Z.
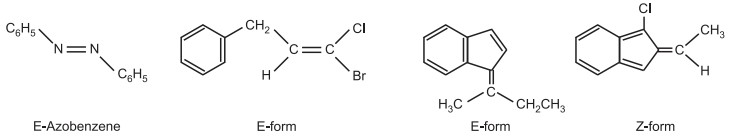
(iii) Syn/Anti system of Nomenclature: The cis-trans isomerism in some classes (such as oximes, diazoates, and azo) containing one or more carbon to nitrogen or nitrogen to nitrogen double bonds is designated by syn/anti-isomerism. Syn/anti nomenclature is based upon two substituents in an acyclic molecule. For example, in stereoisomeric oxime, the configuration of oximes is usually denoted by prefixes “syn” and “anti” instead of cis and trans.
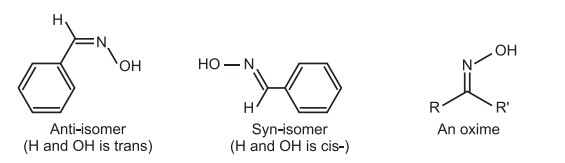
The oximes may be of two types.
(a) Aldoximes: These are derived when aldehydes are treated with hydroxyl amine. (either R or R’ is hydrogen), and
(b) Ketoximes: These are derived when ketones are treated with hydroxyl amine (both R or R’ are alkyl/aryl groups).
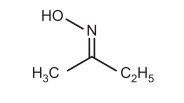
The constrained rotation of the C = N bond is what causes the geometric isomerism in oximes. The hydrogen atom and the hydroxyl group are on the same side of the C = N in syn-aldoximes, whereas they are on the opposite side in the anti-form. The syn and anti descriptors, on the other hand, describe the spatial connection between the group—whose name appears first in the compound name—and the hydroxyl group in the case of ketoxime. For instance, the name of the butanone ketoxime might be either syn methyl ethyl ketoxime or anti-ethyl methyl ketoxime (methyl and OH are syn) (ethyl and OH are anti). The syn acetaldoxime is known as E-acetaldoxime and the anti-form is known as Z-acetaldoxime according to the E-Z notation.
Similarly, Syn/Anti nomenclature is also used for octahedral complex fused rings. The syn-isomer has adjacent fused rings whereas the anti-isomer has opposite fused rings.
Asymmetric synthesis