The concepts of immunogenicity and antigenicity are critical to the understanding of adaptive immunity. By definition, an immunogen is a molecule that stimulates the immune system to produce a response. An antigen is the part of the immunogen that reacts with immune effector cells or soluble antibodies. The term allergen is used to denote an immunogen that elicits the production of allergic antibodies.
Table of Contents
Characteristics of an immunogen
Foreignness
Immunogens that are considered “self ” do not evoke an immune response; thus, foreignness is a critical attribute of immunogens. Phylogenetic differences between the host and the immunogen determine foreignness. For example, a vigorous response is generated when human serum albumin is injected into a mouse. In contrast, only a minimal immune response is observed when mouse albumin is injected into a rat.
Internal Rigidity and Tertiary Structure of an immunogen
Most immunogens are proteins, which are complex rigid structures with a conformation defined by primary, secondary, and tertiary structures. Carbohydrates are composed of linear, repeating carbohydrate units with minimal structural rigidity. Therefore, carbohydrates are generally poor immunogens. The immunogenicity of deoxyribonucleic acid (DNA) depends on molecular weight and the extent of methylation. High-molecular-weight hypomethylated DNA is immunogenic. Other forms of DNA do not evoke an immune response.
Lipids, which are linear carbon chains with no defined tertiary structure, are rarely immunogenic because they lack structural rigidity. The exception is cardiolipin which is used as an antigen in the Wassermann test for syphilis
Size of an immunogen
Size is an important determinant for antigenicity. Molecular structures less than 3000 MW (molecular weight) do not elicit an immune response, whereas maximum immune system stimulation is achieved with large antigens (e.g., 100,000 MW). Large macromolecules are better immunogens because they are insoluble and more easily ingested and processed by macrophages for presentation to lymphocytes.
Degradability Size of an immunogen
Immunogens must be degradable by macrophages to stimulate an immune response, antigen-presenting cells must process the immunogen to yield small polypeptides between 7 and 30 amino acids. The small polypeptides are presented to lymphocytes.
Biologic factors influencing immunogenicity
Genetics
Genetics influences a person’s ability to respond to specific immunogens. Genetic nonresponsiveness is the result of two defects. Some individuals lack a lymphocyte clone with a T cell receptor (TCR) directed at the antigen. Other individuals have a defect in antigen processing and cannot present the antigen to T cells.
Age
The immune response is influenced by a person’s age. Infants are born with a still-developing immune system and cannot mount an immune response to some antigens. Infants are protected by maternal antibodies that cross the placenta before birth and by antibodies in breast milk after birth. An infant’s immune system becomes fully functional between 6 and 12 months of age.
On the other end of the spectrum, the functional capacity of the immune system wanes with age, and older adults have a reduced ability to mount an effective immune response.
Ancillary factors influencing immunogenicity
Concentration
The immune response is a reflection of immunogen concentration and follows a bell-shaped curve, referred to as gaussian distribution. Small amounts of antigen fail to stimulate the immune system and induce an irreversible tolerance to the antigen. Excessively large concentrations paralyze the antigenpresenting cells. Only optimal concentrations on the bellshaped curve generate an immune response.
Route of Administration
The magnitude and the nature of the immune response are determined by the route of administration. Subcutaneous or intramuscular administration stimulates the systemic immune system and protects the host from dying of the disease. Slow release of the antigen from the subcutaneous depot stimulates the immune system maximally. When the antigen is administered by the mucosal route, the host is protected from infection and mortality from the infection. Oral administration is less effective because of rapid clearance of the antigen from the circulation or inactivation of the antigen by gastric fluids.
Epitopes
The molecular fragment of an antigen that interacts with effector cells or antibodies is called an epitope, or antigenic determinant. Proteins are usually large, complex structures that have multiple and different epitopes. One epitope, however, is dominant in the elicitation of an immune response. Epitopes are either linear or conformational. Proteins have both linear and conformational epitopes. Linear epitopes comprise six to eight contiguous amino acids in the primary amino acid sequence of a polypeptide (Figure 3-1). Lymphocyte receptors or antibodies recognize linear epitopes in the native, fragmented, or extended conformations of the polypeptide. In contrast, conformational epitopes are created when protein segments are folded into a tertiary structure. The immune system recognizes the native conformational epitopes or the isolated fragments that retain the appropriate conformational tertiary structure.
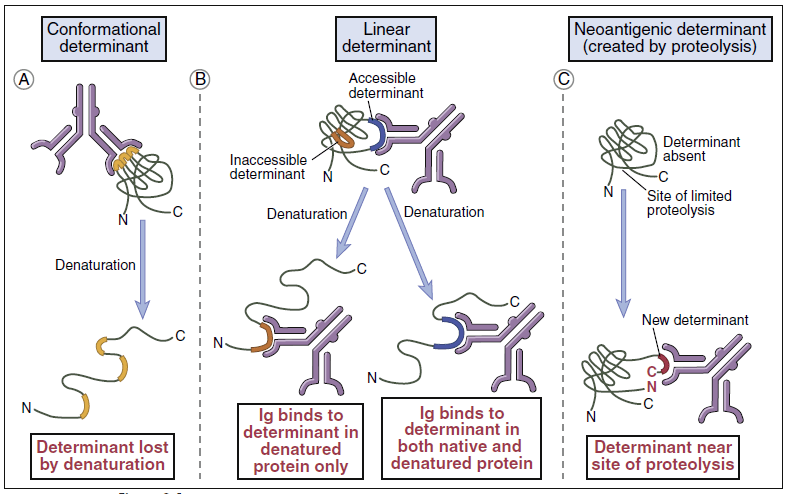
depend on protein folding (conformation) as well as on primary structure.(Immunogenicity)
T cell and b cell recognition of epitopes
Linear epitopes are recognized by T cells. Often, these epitopes are internal hydrophobic amino acid sequences processed by macrophages and presented to T cells in the context of human leukocyte antigen (HLA) class I and class II molecules. Processed epitopes containing 7 to 17 amino acids are presented to T lymphocytes by antigen-presenting cells. B cell epitopes from globular proteins, which range from 5 to 30 amino acids, are usually conformational. The length and flexibility of the epitope ensure high-affinity bonding to B cell receptors or circulating antibodies.