Table of Contents
Antigen
The antigen is a chemical that can activate lymphocytes, the body’s infection-fighting white blood cells, and stimulate an immunological response. Antigens are divided into two categories: foreign antigens (or heteroantigens) and autoantigens (or self-antigens). Antigens that come from outside the body are called foreign antigens.
Types of antigens
White Blood Cell Alloantigens
White blood cells express alloantigens, which are part of the body’s self-recognition system. Alloantigens are found in some, but not all, members of a species. In mice, the genes for white cell alloantigens are localized in the MHC on chromosome 17. Humans have a similar locus called the human leukocyte antigen (HLA) complex located on chromosome 6. These glycoproteins are subdivided into class I and class II antigens. These antigens are involved in the presentation of antigen, the rejection or acceptance of grafts between members of the same species (e.g., allografts), or both. Class I HLA antigens are constitutive and are expressed on all nucleated cells. Class II antigens are inducible and are only expressed on macrophages and monocytes. Multi-parous women and transplant recipients often develop antibodies directed at alloantigens.
Transfusion-Related Acute Lung Injury
Transfusion-related acute lung injury (TRALI) is caused by the transfusion of blood products containing anti-HLA antibodies. The antibodies react with HLA molecules on circulating neutrophils, which pool in the lung capillaries and move into extravascular spaces. Antibody-coated neutrophils release free oxygen radicals, enzymes, and arachidonic acid metabolites, which damage the alveolar epithelium. Leakage of fluid into alveoli from capillaries causes pulmonary edema. Patients present with shortness of breath, hypoxia, and fever.
Red Blood Cell Antigens
The best-studied antigens in humans are found on red blood cells. Red blood cell antigens are water-soluble glycopeptides consisting of heterosaccharides attached by a glycosidic linkage at the reducing ends. The common core structure consists of a galactose and N-acetyl glucosamine attached to a glycoprotein core and is called the H-antigen. Most individuals have a fucosyltransferase enzyme that attaches fucose to the terminal end of the H antigen. Two variants of a glycosyltransferase enzyme add additional sugars to the H antigen.]
The type A antigen is created when N-acetylgalactosamine is added to the terminal galactose. When an additional galactose is added to the terminal sugar, it produces a type B red cell antigen. In individuals who are heterozygous for blood group antigens, galactosamine and N-acetylglucosamine are added to the core H antigen, which creates the AB blood type (Figure 3-2).
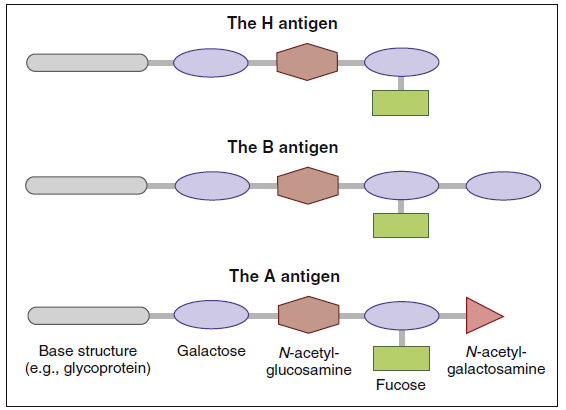
Using the ABO isoantigens, it is possible to classify red blood cell types into populations of universal donors or universal recipients. Since the O type contains an epitope that is common to all red blood cells (H antigen) and is non-immunogenic, individuals are universal donors. Conversely, persons with type AB blood are considered universal recipients because they express both A and B antigens. When transfused with type O, A, or B blood, persons with type AB blood do not mount an immune response because neither the A antigen nor the B antigen is foreign.
Antibodies directed at non-self blood group antigens are often present in serum. These natural antibodies are formed because A and B antigens are found in a wide variety of unrelated plant and animal tissues. Ingestion of these heterologous antigens stimulates the production of antibodies directed at non-self red cell antigens. For example, antibodies directed at blood group A are present in the serum of people with type B red blood cells. Conversely, persons with blood group A have anti-B antibodies in the serum. Antibodies directed at ABO blood group antigens can also be generated as a consequence of previous pregnancies, transfusions, or organ transplantations.
Hemolytic Transfusion Reactions
Hemolytic transfusion reactions occur when a patient receives red blood cells with major or minor antigens to which they have antibodies. An ABO mismatch typically occurs when a patient with group O blood type is transfused with group A, B, or AB blood cells. Antibodies in the recipient’s blood react with red cell isoantigens and activate a complement that lyses the recipient’s red blood cells. As a result, hemoglobin is found in blood and urine, and microthrombi are formed. These small thrombi localize in the capillaries of the hands and feet. Obstruction of blood flow in the capillaries causes tissue necrosis and gangrene. Renal failure and cardiovascular collapse are other dangerous sequelae in these patients.
Treatment of Hemolytic Transfusion Reactions
Patients undergoing hemolytic transfusion reactions may experience mild or severe reactions. Mild symptoms include rashes, fever, and back pain. Acute kidney failure is a significant and severe problem in some patients. Treatment is directed at reducing the mild symptoms, increasing renal blood flow, and preserving urinary output (Table 3-1).
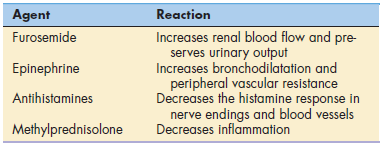
Experiencing Mild or Severe Hemolytic
Transfusion Reactions
Rhesus Factor Isoantigens
Rhesus factor (Rh or RhD), or Rhesus antigen, is another isoantigen found on red blood cells. The name is derived from the fact that the blood antigen was first described in the Rhesus monkey. Rh antigens are nonglycosylated, hydrophobic cell membrane antigens expressed in 85% of the human population (Rh-positive, or Rh+). Individuals with alterations or a deletion of the Rh protein are considered Rh negative (Rh–). If individuals with Rh– blood are exposed to Rh+ antigens, a vigorous antibody response is evoked. An Rh mismatch during transfusion causes a unique extravascular hemolytic anemia. Antibodies directed at the Rh factor do not activate complement. Hence, no intravascular hemolysis occurs. Rather, red cells are coated with antibodies and removed by splenic macrophages.
Rh antigens play a significant role in transfusion reactions; however, Rh compatibility takes on an even more significant and crucial role in pregnancy. Serious problems arise when the Rh– mother is exposed to Rh+ cells. Exposure can occur as a consequence of normal delivery, trauma, or blood transfusions. Within 30 days of exposure, the mother will develop anti-Rh antibodies. However, these large (900,000 MW) IgM antibodies cannot cross the placenta. Therefore, the first child will be unaffected. If the mother is exposed to Rh T cells during a second pregnancy, small (150,000 MW) IgG antibodies are produced. These antibodies can cross the placenta and attack the fetal red blood cells, causing autoimmune hemolytic anemia, called erythroblastosis fetalis, with severe consequences. The lysis of red cells liberates hemoglobin, which is converted to bilirubin. Accumulation of bilirubin damages the central nervous system, and the infants develop hypotonia, hearing loss, and intellectual disabilities.
Severe forms of erythroblastosis fetalis are characterized by cardiac failure, pericardial effusions, and edema (hydrops fetalis).
Treatment of Rh Incompatibility
Pooled human anti-D immune globulin (Rh IgG or RhoGAM) treatment is indicated at 28 weeks and within 72 hours after delivery if the baby is Rh+. It is also indicated following spontaneous or induced termination and any event that could lead to transplacental hemorrhage. A single dose (50 micrograms [μg]), which is administered following first-trimester pregnancy termination, is enough to neutralize 2.5 mL of fetal blood. A 300-μg dose, which can neutralize 15 mL of fetal blood, is administered for all other indications. When properly administered, the incidence of adverse effects is less than 0.5%.