The boiling point of water increases when the external pressure is increased whereas decreased external pressure decreases the boiling point. This principle is used in the process of freeze-drying. Many compounds cannot be distilled at atmospheric pressure because their boiling points are so high. At their normal boiling points, the compounds decompose. Thus, some of these materials can be distilled under reduced pressure because the required temperature to boil the liquid can be lowered significantly. If the boiling point is lowered by 10 °C each time the external pressure is halved. To vaporize a liquid, its temperature can be raised or its pressure can be decreased. Distillation under reduced pressure can also be called as vacuum distillation.” During vacuum distillation, the pressure inside the distillation column is maintained at a vacuum to lower the temperature needed to vaporize the liquid. This method of distillation is used for heat-sensitive products, liquids with low viscosities, and liquids that tend to foul or foam. In vacuum distillation, vacuum pumps are added to the distillation system to decrease the column pressure below atmospheric pressure, as shown in the figure below. Careful pressure control is important because the separation is dependent on the differences in relative volatility at a given temperature and pressure. Changes in relative volatilities could adversely affect the separation.
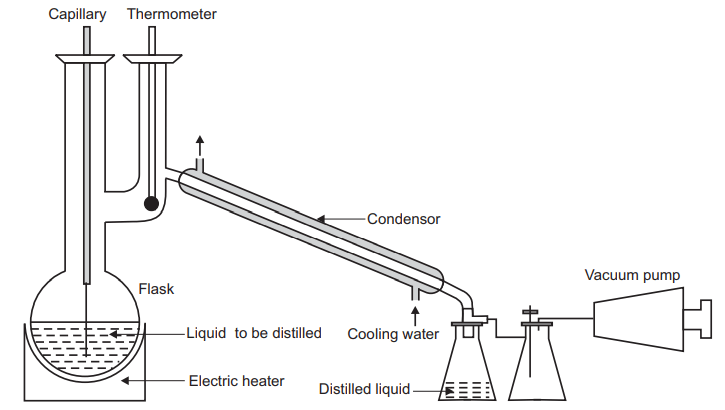
Table of Contents
Principle for Distillation under reduced pressure
This distillation method works on the principle that boiling occurs when the vapour pressure of a liquid exceeds the ambient pressure. Vacuum distillation is used with or without heating the mixture.
Construction for Distillation under reduced pressure
The vacuum distillation unit consists of a distillation column, condensing distillate, and reboiler. Vacuum pumps and vacuum regulators are added to distillation columns to maintain the column at a vacuum. Many mixtures can be distilled at much more economical temperatures with the use of these vacuum distillation columns.
Working for Distillation under reduced pressure
Vacuum distillation is also called as low-temperature distillation. The target product in this distillation could either be the remaining product, the distilled product or a purified product. The vacuum pressure associated with a distillation depends on the product to be distilled. For example, volatile substances like those used in oil refineries are likely to undergo vacuum distillations at above 1 Torr, perhaps 20-50 mmHg.
Applications for Distillation under reduced pressure
(i) The products of normal distillation are further distilled using vacuum distillation. The high boiling point hydrocarbons, such as lubricants and waxes, are separated at economical temperatures.
(ii) Vacuum distillation is also used in the separation of sensitive organic chemicals and recovery of organic solvents.
Advantages
(i) Vacuum distillation reduces the number of stages needed in distillation.
(ii) The product output per day is very high.
(iii) It increases the relative volatility of the key components in many applications. Lower pressures increase relative volatilities in most systems.
(iv) It requires lower temperatures at lower pressures.
(v) Vacuum distillation can improve a separation by prevention of product degradation because of reduced pressure leading to lower tower bottoms temperatures.
(vi) It has a very high capacity to handle liquid mixtures giving the high yield and highest purity.
(vii) It requires low capital cost, at the expense of slightly more operating cost. Using vacuum distillation one can reduce the height and diameter, and thus the capital cost of a distillation column.
(viii) Columns can be operated at lower temperatures.
Disadvantages
(i) High energy costs of vacuum pumps.
(ii) Pressure and energy losses due to any leaks or cracks.
(iii) Large column diameters needed for the process to be efficient.