Historically, distillation has been used since 1200 BC in perfumery operations. Early forms of distillation were batch processes using one vaporization and one condensation. Purity was improved by further distillation of the condensate. Greater volumes were processed by simply repeating the distillation. Chemists were reported to carry out as many as 500 to 600 distillations in order to obtain a pure compound. In the early 19th century, the basics of modern techniques including pre-heating and reflux, were developed. In 1877 U.S. Patent was granted for a tray column for distillation of ammonia and in the subsequent years for oil and spirits. With the emergence of chemical engineering as a discipline at the end of the 19th century, scientific rather than empirical methods were applied. More accurate designs were used during the development of the petroleum industries. Today availability of powerful computers has allowed direct computer simulation of distillation columns.
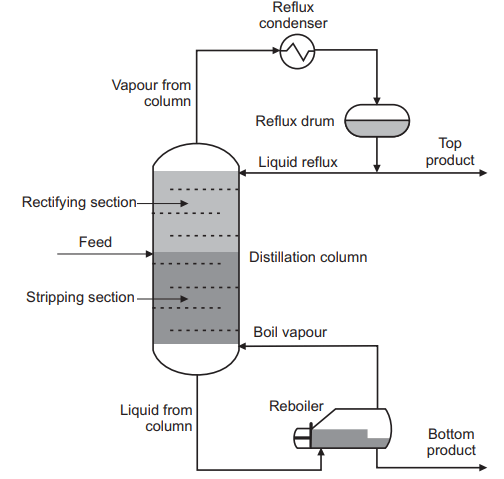
Table of Contents
What is Distillation?
Distillation is a process by which a liquid mixture is separated into fractions with higher concentrations of certain components by exploiting differences in relative volatility. Distillation has been used widely to separate volatile components from non-volatile compounds. In industrial settings such as oil refineries and natural gas processing plants, this separation process is undertaken using a distillation column.
Distillation is one of the most important processes for separating the components of a solution. The solution is heated to form a vapor of the more volatile components in the system, and the vapor is then cooled, condensed, and collected as drops of liquid, Shown in the Figure above. By repeating vaporization and condensation, individual components in the solution can be recovered in a pure state. Essences and many pure products from the oil refinery industry are processed via distillation.
Objective of Distillation
The basic objective of distillation is to separate the liquid mixture into two or more components. In a basic distillation column, a feed stream enters in the middle of the column and two streams leave, one at the top and one at the bottom. Components with lower boiling points are concentrated in the stream leaving the top while components with higher boiling points are concentrated in the stream leaving the bottom. Separation is achieved by controlling the column temperature and pressure to take advantage of differences in the relative volatility of the mixture components and therefore tendency to change phase. The lighter, lower boiling point components evaporate and travel up the column to form the top product, and the heavier, higher boiling point components condense and travelling down the column to form the bottom product.
Basic principles of distillation
The mechanism involved in distillation is the differences in volatility between individual components. With sufficient heat applied, vapors are formed from the liquid solution. The liquid product is subsequently condensed from the vapor phase by the removal of the heat. Therefore, heat is used as the separating agent during distillation. In general, distillation can be carried out either with or without reflux involved. For the case of single-stage differential distillation, the liquid mixture is heated to form a vapor that is in equilibrium with the residual liquid. The vapor is then condensed and removed from the system without any liquid allowed to return to the still pot. This vapor is richer in the more volatile component than the liquid removed as the bottom product at the end of the process. However, when products of much higher purity are desired, part of the condensate has to be brought into contact with the vapor on its way to the condenser and recycled to the still pot. This procedure can be repeated many times to increase the degree of separation in the original mixture. Such a process is normally called “rectification.”
Fractionation: It is another term for distillation also called fractional distillation.
Feed: The liquid and/or gas feed into the distillation column.
Feed tray: The tray below the inlet nozzle is called the feed tray.
Heavy Component: The component with the lower relative volatility, for example, simple hydrocarbon, is a component with a higher molecular weight. It is found in higher concentrations in the bottom product of the column.
Light Component: The component with the higher relative volatility, for example, simple hydrocarbon, is a component with a lower molecular weight. It is found in higher concentrations at the top of the column.
Stripping section: It is a section that consists of trays between the bottom of the column and the feed tray. In the stripping section, the aim is to concentrate the heavier component in the liquid phase.
Rectifying section: It is a section that consists of trays between the feed tray and the top of the column. In the rectifying section, the aim is to concentrate the lighter component in the vapor phase.
Top Product: It is a product which leaves from the top of the column, also called the distillate. This product is usually passed through a heat exchanger and liquefied.
Bottom Product: It is the product that leaves through the bottom of the distillation column.
Reflux: A portion of vapor from the top of the column has been condensed to a liquid and returned to the column as a liquid above the top tray.
Reboiler: A heat exchanger at the bottom of the column which boils some of the liquid leaving the column. The vapor generated returns to the column at the bottom of the stripping section.
Vapor-liquid equilibrium (VLE) Curve: A plot of the actual composition of the lighter component in the vapor phase for a given composition in the liquid phase. Usually, it is derived from thermodynamic data.
Zeotropic mixture : It is a mixture of liquids with different boiling points. For example, nitrogen, methane, ethane, propane etc.
Azeotropic mixture: It is a mixture of two or more liquids that has a constant boiling point because vapor has the same composition as a liquid mixture.
Principle of Separation
Distillation takes advantage of the difference in relative volatility of the feed mixture components. Generally for two or more compounds at a given pressure and temperature, there will be a difference in the vapor and liquid compositions at equilibrium due to component partial pressure. Distillation exploits this by bringing liquid and gas phases into contact at temperatures and pressures that promote the desired separation. During this contact the components with the lower volatility (typically lower boiling point) preferentially moves into the liquid phase while more volatile components move into the vapor phase.
A distillation column may use either trays or a packed bed to bring the gas and liquid into contact. For a column using trays, we can consider the changes to gas and liquid phase compositions as they both enter and exit a single tray. The liquid entering the tray will contact the gas exiting the tray, Shown in the Figure below. The hotter vapor phase heats the incoming liquid phase as it bubbles through the tray, evaporating the light components which then leaves the tray with the vapor phase. Conversely, the cooling of the vapor phase by the liquid phase will causes the heavier components of the vapor phase to condense and exit the tray with the liquid phase.
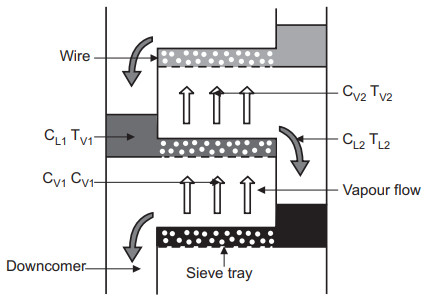
For the liquid across the tray:
CL1,heavy < CL2,heavy
CL1, light > CL2,light
TL1 > TL2
For the vapor through the tray:
CV1,heavy > CV2,heavy
CV1, light < CV2,light
TV1 > TV2
Where C is concentration and T is temperature.
When packing is used rather than trays the principle remains the same, in fact, packing is often referenced in terms of the height equivalent to a theoretical plate (HETP) i.e. what height of packing is equivalent to one theoretical plate. The packing is just an alternative method to bring the liquid and vapor phases into contact with the liquid generally flowing over the surfaces of the packing material, while the vapor passes up through the space between packing elements.
Typical Operating Parameters
The distillation process can be improved by understanding the following operating parameters.
Temperature
The basic temperature profile of a distillation column is hotter at the bottom and cooler at the top. For a simple two-component distillation the temperature at the bottom is just lower than the boiling point of the heavier component. The temperature at the top of the column is just above the boiling point of the lighter component. In order to have a heavy component remain as a liquid at the bottom of the column and the lighter component stay as a gas, we set the temperature at the bottom to match this requirement. This temperature is set by adding heat via a heat exchanger. Typically the heat added to the bottom of the column is easy to control, via steam or hot oil flow rates.
At the top of the column, the situation is reverse. The light component remains as gas while the heavier component is condensed to a liquid and falls back down the column. The top temperature is set just above the boiling point of the lighter component. The temperature control situation is different at the bottom of the column because the top product is to be a liquid when we send it for storage. All of the gas coming out of the top of the column is condensed to a liquid. This liquid stream is split with some returning to the column and some going to storage. The top temperature is often controlled by changing the reflux rate, i.e. the flow rate of liquid sent back to the top of the column. A higher reflux rate means cooler liquid falling down the column against the rising warmer gas, and the top temperature is lower. Overall heat is added at the bottom of the column and heat is extracted at the top of the column. Inside the column, the temperature balance is created between the hot gas rising up the column and the cooler liquid falling down the column.
Pressure
There is typically a pressure gradient across the column with the pressure being higher at the bottom of the column than the top. This pressure gradient occurs as the liquid coming down the column hampers the flow of vapor up the column and imposes a pressure loss on the flow. In steady-state distillation, the pressure in the column is held constant, and the temperature is varied to control the composition of the product streams.
Applications
(i) Distillation is used for many commercial processes, such as the production of gasoline, distilled water, xylene, alcohol, paraffin, kerosene, and many other liquids.
(ii) Distillation is used for purifying solvents and liquid reaction products.
(iii) It is used in the manufacturing of distilled water, double distilled water used in Water for Injection, and other pharmaceutical preparations.
(iv) Toxic and costly organic solvents are used in the extraction, synthesis, and analysis of drugs. This solvent can be recovered by distillation for economic as well as environmental protection benefits.
(v) Distillation is used in the separation of volatile oils such as clove oil, anise oil, cardamom oil, eucalyptus oil, etc. from the plant extracts.
(vi) It can be used in the separation of volatile components from a mixture of two or more volatile liquids.
(vii) It can be used as a quality control method for alcohol content in liquid formulations. The alcohol is separated from formulations by distillation and alcohol content is determined.
(viii) It can be used to liquefy and separate gases from the air. For example, nitrogen, oxygen, and argon are distilled from the air.
(ix) Distillation is used in crude fermentation broths to separate alcoholic spirits.
(x) It can also be used in the fractionation of crude oil into gasoline and heating oil.
Make sure check our amazing post on: Steam Jacketed Kettle