Quantum theory and Bohr atom model
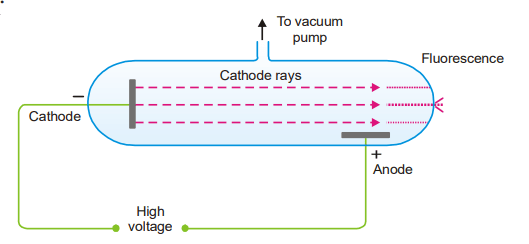
Bohr atom model Rutherford’s model laid the foundation of the model picture of the atom. The Rutherford model, on the other hand, provided no information on the position of the electrons or how they were organized around the nucleus. Rutherford saw that electrons were circling the nucleus. However, according to classical physics, an electron traveling … Read more