Determination of the charge on an electron: R.A. Milikan (1908) used what is known as the Milikan’s Oil-drop Experiment to determine the absolute value of an electron’s charge. The equipment he employed is seen in the Figure given below. He poured oil droplets into the device using an atomizer. Through a hole in the upper plate, an oil droplet falls. The air between the plates is then subjected to X-rays, which cause the air molecules to expel electrons. The oil droplet captures some of these electrons, giving it a negative charge. The droplet falls under the influence of gravity when the plates are earthed.
He adjusted the strength of the electric field between the two charged plates so that a particular
oil drop remained suspended, neither rising nor falling. At this point, the upward force due to the
negative charge on the drop just equaled the weight of the drop. As the X-rays struck the air
molecules, electrons are produced. The drop captures one or more electrons and gets a negative
charge, Q. Thus, Q = me
where n = number of electrons and e = charge of the electron. From measurement with different drops, Milikan established that electron has the charge – 1.60 × 10-19 coulombs.
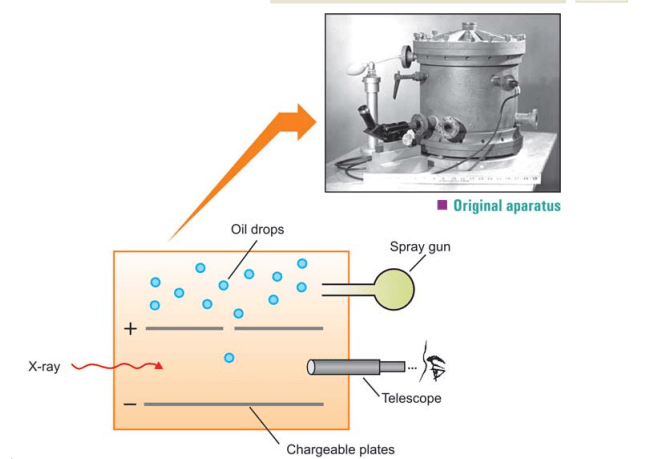
Table of Contents
Mass of Electron
By using Thomson’s value of e/m and Milikan’s value of e, the absolute mass of an electron can be found.
e/m = – 1.76 × 108 coulomb/g (Thomson)
e = – 1.60 × 10-19 coulomb (Milikan)
∴ e/e/m=1.60 × 10-19 /1.76 × 108
Hence m = 9.1 × 10– 28 g or 9.1 × 10– 31 kg
Mass of an Electron relative to H
Avogadro number, the number of atoms in one gram atom of any element is 6.023 × 1023 . From this, we can find the absolute mass of the hydrogen atom.
Mass of 6.023 × 1023 atoms of hydrogen = 1.008 g
∴ Mass of a hydrogen atom =1.008/6.023 × 1023 g
= 1.67 × 10-24 g
But mass of electron = 9.1 × 10-28 g
mass of H atom/mass of electron 1.67 × 10-24/9.1 × 10-28
= 1.835 × 103 = 1835
Thus an atom of hydrogen is 1835 times as heavy as an electron.
In other words, the mass of an electron is 1/1835th of the mass of a hydrogen atom.
Definition of an electron
Having known the charge and mass of an electron, it can be defined as An electron is a subatomic particle which bears charge – 1.60 × 10–19 coulomb and has mass 9.1 × 10-28 g.
Alternatively, an electron may be defined as A particle that bears one unit negative charge and mass 1/1835th of a hydrogen atom. Since an electron has the smallest charge known, it was designated as a unit charge by Thomson.
Make sure check our amazing article related to this post: Measurement of e/m for electrons
