Table of Contents
Protons
Protons are positively charged particles that help make up the atom. Protons are located in the nucleus of the atom (along with neutrons)
E. Goldstein (1886) discovered protons in the discharge tube containing hydrogen.
H ⎯⎯→ H+ + e- (proton)
It was J.J. Thomson who studied their nature. He showed that :
(1) The proton has a mass of 1.672 × 10-24 gramme. On a relative scale, one atomic mass unit equals one proton (amu).
(2) The electrical charge of the proton is equivalent to that of the electron but in the opposite direction. As a result, a proton has a charge of +1.60 × 10-19 coulombs, or + 1 elementary charge unit. The proton was considered to be a unit present in all other atoms since it was the lightest positive particle discovered in atomic beams in the discharge tube. Protons were also discovered in a number of nuclear processes, implying that protons are found in all atoms.

Thus a photon is defined as a subatomic particle that has a mass of 1 amu and charge + 1 elementary charge unit.
A proton is a subatomic particle that has one unit mass and one unit positive charge.
Neutrons
The third subatomic particle was identified in 1932 by Sir James Chadwick. He fired a stream of alpha particles(2 4He) towards a target made of beryllium. A new particle had been expelled, he discovered. It is approximately the same mass as a proton (1.674 × 10-24 g) and has no charge.
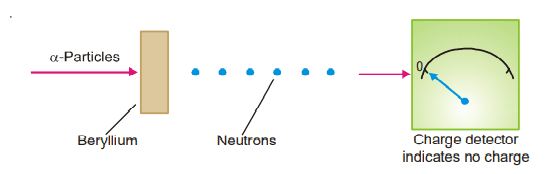
whereby the electric charge detector remains unaffected.
He named it a neutron. The assigned relative mass of a neutron is approximately one atomic mass unit (amu). Thus :
A neutron is a subatomic particle that has a mass almost equal to that of a proton and has no charge.
The reaction which occurred in Chadwick’s experiment is an example of artificial transmutation where an atom of beryllium is converted to a carbon atom through the nuclear reaction.![]()
Subatomic particles
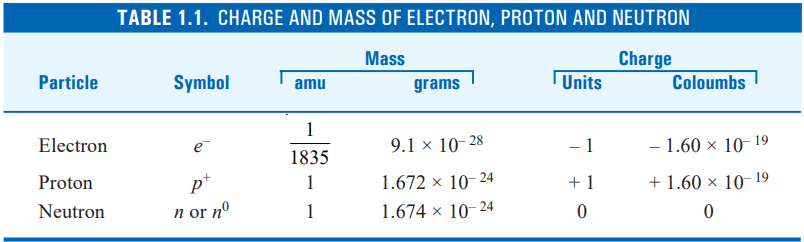
The characteristics of the three main basic particles of the atom, the electron, proton, and neutron, have been investigated previously. These are summed up in the table below.
Nearly all of the ordinary chemical properties of matter can be examined in terms of atoms
consisting of electrons, protons, and neutrons. Therefore for our discussion, we will assume that the atom
contains only these three principal subatomic particles.
Other Subatomic Particles
Many additional subatomic particles, such as mesons, positrons, neutrinos, and antiprotons, have been found in addition to electrons, protons, and neutrons. A slew of new subatomic particles with names like quarks, pions, and gluons have emerged as a result of recent studies. The image of atomic structure grows more complicated with each new discovery. Fortunately, chemists may still use the three-particle model of the atom (electron, proton, and neutron).
Alpha particles
Alpha particles are shot out from radioactive elements at very high speed. For example, they come from radium atoms at a speed of 1.5 × 107 m/sec. Rutherford identified them to be di-positive helium ions, He2+ or 2 4He. Thus an alpha particle has a 2+ charge and 4 amu mass. α-Particles are also formed in the discharge tube that contains helium, He⎯⎯→He2+ +2e_ It has twice the charge of a proton and about 4 times it’s mass.
Conclusion
Despite the fact that the -particle is not an atom’s basic particle (or subatomic particle), Rutherford believed that because of its high energy (1/2mv2), he could fire them like bullets at atoms and so gain information about the structure of the atom.
(1) When H+ ions or protons were generated, he attacked nitrogen and other light elements with -particles. This revealed that protons may be found in atoms other than hydrogen.
(2) The bombardment of thin metal foils gave him a hint of the presence of a positive nucleus in the atom.