Table of Contents
Moseley’s law
Moseley’s law is an empirical law relating to atoms’ distinctive x-rays. Henry Moseley, an English physicist, developed and published the law in 1913-1914.
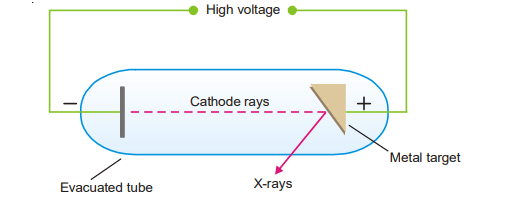
Hitherto atomic number was designated as the ‘position number’ of a particular element in the Periodic Table. Moseley found that when cathode rays struck different elements used as anode targets in the discharge tube, characteristic X-rays were emitted. The wavelength of these X-rays decreases in a regular manner in passing from one element to the next one in order in the Periodic Table.
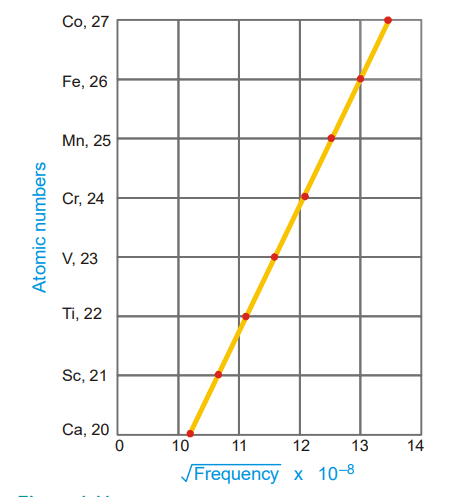
Mosley found a straight line by plotting the atomic number against the square root of the frequency of the X-rays emitted, indicating that the atomic number was not only a “position number,” but a basic characteristic of the atom. He also proposed that the number of positive charges or protons in the nucleus was connected to the wavelength (or frequency) of the emitted X-rays. The wavelength varied on a regular basis as each element in the Periodic Table gained a new one. One proton (atomic mass unit) is greater than the preceding one. Moseley determined the number of positive charge units on the nuclei of various atoms and found that:
Atomic Number of an element is equal to the number of protons in the nucleus of the atom of that element.
Since the atom as a whole is electrically neutral, the atomic number (Z) is also equal to the number of extranuclear electrons. Thus hydrogen (H) which occupies first position in the Periodic Table has atomic number 1. This implies that it has a nucleus containing one proton (+ 1) and one extranuclear electron (– 1).
Now the term Atomic Number is often referred to as the Proton Number.
What is mass number ?
The total number of protons and neutrons in the nucleus of an atom is called the Mass Number, A, of the atom.
In situations where it is unnecessary to differentiate between protons and neutrons, these elementary particles are collectively referred to as nucleons. Thus mass number of an atom is equal to the total number of nucleons in the nucleus of an atom.
Obviously, the mass number of an atom is a whole number. Since electrons have practically no mass, the entire atomic mass is due to protons and neutrons, each of which has a mass almost exactly one unit. Therefore, the mass number of an atom can be obtained by rounding off the experimental value of atomic mass (or atomic weight) to the nearest whole number. For example, the atomic mass of sodium and fluorine obtained by experiment is 22.9898 and 26.9815 amu respectively. Thus their mass numbers are 23 for sodium and 27 for fluorine.
Each different variety of atom, as determined by the composition of its nucleus, is called a nuclide.
Composition of the nucleus
Knowing the atomic number (Z) and mass number (A) of an atom, we can tell the number of protons and neutrons contained in the nucleus. By definition :
Atomic Number, Z = Number of protons
Mass Number, A = Number of protons + Number of neutrons
∴ The number of neutrons is given by the expression :
N = A – Z
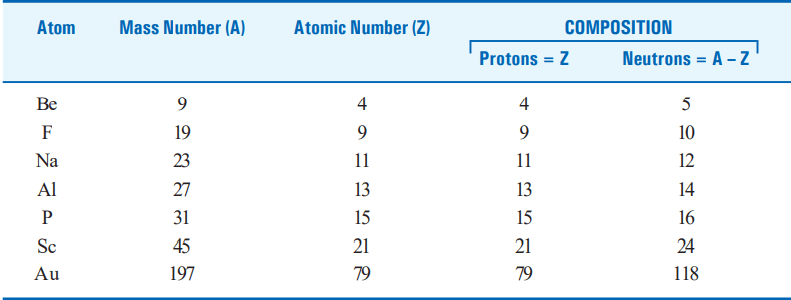
COMPOSITION OF THE NUCLEUS OF SOME ATOMS
Make sure check our amazing article: Rutherford’s atomic model – the nuclear atom