When a source producing lines is put in a high magnetic field, spectral lines are broken up into components, as found by a scientist named Zeeman in 1896. The effect is known as the Zeeman effect after its discoverer. The Zeeman effect was observed using the apparatus depicted in the diagram below.
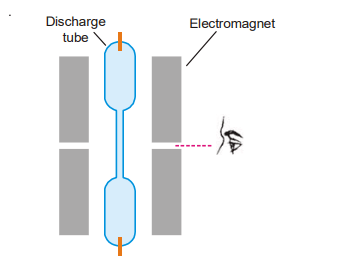
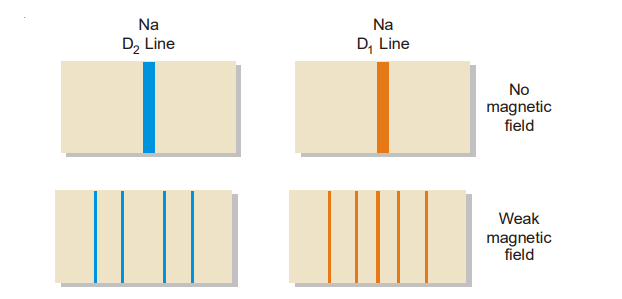
It comprises electromagnets capable of creating a high magnetic field, as well as pole pieces with longitudinal holes. Between the pole pieces, insert a discharge tube or a sodium vapor lamp that emits radiation. When the spectral lines are viewed axially through the hole in the pole pieces, parallel to the magnetic field, they split into two components, one with a shorter wavelength (higher frequency) and the other with a longer wavelength (lower frequency) than the original spectral line, which is no longer visible. The two lines are symmetrically situated around the position of the original line and the change in wavelength is termed the Zeeman shift (denoted as dλ). When viewed in a direction perpendicular to the applied field the lines split up into three, the central one having the same wavelength and frequency as that of the original line and the other two occupying the same position as observed earlier.
To explain the Zeeman effect, let us consider the motion of an electron in a particular orbit corresponding to its permitted angular momentum. The motion of the electron in an orbit is equivalent to a current in a loop of wire. If a current-carrying loop of wire is placed in a magnetic field, it experiences a torque, and the energy of the system depends upon the orientation of the loop concerning the magnetic field. The correct values of the energies are obtained if the components of the angular momentum of the electron along the direction of the magnetic field are restricted to the value.
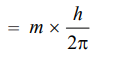
where m = 0, ± 1, ± 2, … and so on. Corresponding to these values of m, a given line splits into as many lines. Hence for each frequency of radiation emitted by the atom in the absence of a magnetic field, there are several possible frequencies in the presence of it. This is, in fact, the cause of the Zeeman Effect.
The shift in the frequency dλ for each of the component lines is given by Lorentz’s theoretically derived equation as to
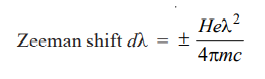
where H is the strength of the magnetic field, e is the electronic charge, m the mass of the electron, c the velocity of light, and λ the wavelength of the original line in the absence of magnetic field. The equation can also be written as
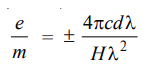
The validity of the above equation can be tested experimentally by observing the Zeeman shift dλ for a given light source of known λ (say D-line of sodium) for a magnetic field of known strength H and calculating the value of e/m for the above equation. Lorentz found that the e/m of the electrons found by this method comes out to be the same as by any other method.
Make sure check our amazing article on: Bohr’s Explanation of Hydrogen Spectrum