Co-stimulatory molecules are a diverse collection of cell surface molecules that work to enhance or neutralize the first activation signals sent to T cells by the TCR when it interacts with an antigen/major histocompatibility complex (MHC), affecting T cell development and destiny. While it was formerly assumed that co-stimulation was required for T cell activation at all phases of development, it is now recognized that the needs for co-stimulation, as well as the co-stimulatory molecules involved, varied depending on the stage of T cell differentiation.
Table of Contents
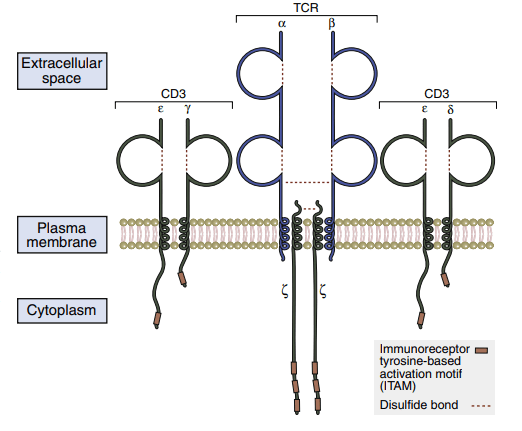
CD3–t cell receptors protein complex
Because of its short cytoplasmic tails, the TCR is incapable of intracellular signaling. The TCR forms the CD3 complex with numerous signaling proteins to transmit activation signals to the nucleus. The pan T cell makerCD3 is made up of five component proteins. Two copies of -chains that form disulfide-linked homodimers (–) are present in the assembly of the CD3 complex. The intracellular domains of the and chains are involved with signal transduction. These chains have 44 to 81 amino acid sequences known as immunoreceptor tyrosine-based activation motifs (ITAMs), which are required for signaling. TCR ζ-chains have a short, nine-amino-acid transmembrane sequence and a long (113 amino acids) cytoplasmic tail with three ITAMs.
Stabilizing molecules
The TCR has a poor affinity for class II compounds. The dissociation constant (Kd) ranges between 10–5 and 10–7M. Additional molecules are required to stabilize the complex due to its poor affinity (showing in the below Figure ). The connection between the TCR and the APCs that express HLA molecules is stabilized by CD4 molecules. CD4Th1 and CD4Th2 cells, macrophages, and dendritic cells all express CD4 molecules. The CD4 molecule features a short transmembrane domain and a cytoplasmic tail that allows it to communicate with the nucleus. The lck or p56 kinases are activated when serine residues in the cytoplasmic tail are phosphorylated. These kinases play a critical role in the activation of HLA class II-restricted T helper cells. CD8 is a dual-chain heterodimer that stabilizes the interaction between CD8 cells and antigen-loaded HLA class I molecules. Extracellular CD8 domains bind to the α3 region class I molecules.
Co-Stimulatory Molecules
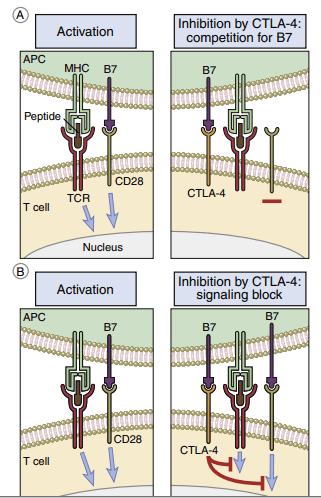
CD28/B7 Interaction
After interacting with B7 molecules on APCs, T cells can be activated or deactivated. Monocytes, macrophages, interstitial dendritic cells, and epithelial dendritic cells all express B7-1 and B7-2 molecules. One of the two B7 isoforms is produced after antigen processing, and this affects the immune response’s character. B7-1 activation stimulates CD4Th2 (helper cells in antibody production), while B7-2 activation activates CD4Th1 (inflammatory response). B7 has two ligands in T cells. T lymphocytes are activated via interactions between CD28 and B7. The second ligand, cytotoxic T lymphocyte protein 4, binds to B7 with a high affinity (CTLA-4). Interaction between CTLA-4 and B7 downregulates T cell signaling and prevents T cell activation( Figure below)
CD40 Ligand/CD40
CD40 ligand (CD40L) is a 261-amino-acid membrane glyco[1]protein expressed on activated CD4 lymphocytes. The expression occurs shortly after T cell activation. The natural ligand for CD40L is CD40, which is a transmembrane protein present on B cells, follicular dendritic cells, and macrophages. CD40– CD40L interactions play an important role in the amplifica[1]tion of the immune response and the production of antibodies. Ligation stimulates the secretion of interleukin 12 (IL-12) from the APCs. In turn, IL-12 activates CD4Th1, CD8, and natural killer (NK) cells and amplifies the immune response.
In the production of antibodies, T and B cells are brought into close proximity. CD40/CD40L ligation between T and B cells induces B cell activation, differentiation, isotypic switch[1]ing, and the generation of memory cells.
CD2/CD58]
CD2 is a glycoprotein present on 90% to 95% of mature T cells and NK cells. Lymphocyte function-associated antigen (LFA-3 or CD58) on APCs is the principal ligand for CD2. Both molecules are adhesion factors that strengthen the inter[1]actions between T cells and APCs. Ligation results in the pro[1]duction of proteins that regulate the cellular response to IL-2.
CD11a/CD18/CD54
Integrins are a family of molecules that facilitate the adhe[1]sion of T cells to molecules on APCs. CD11a is an α1integrin subunit that associates with an β2-integrin subunit (CD18) to form LFA-1. LFA-1 is a glycoprotein expressed on all leuko[1]cytes. The LFA-1 ligand is intercellular adhesion molecule 1 (ICAM-1) on APCs. Interaction between these adhesion facs [1]tors plays a critical role in T cell activation, T cell mobility, and control of autoimmune diseases.
Make sure check article related to this post: Origin of t cell receptor diversity
REFERENCES
Engel I, Letourneur F, Houston JT, Ottenhoff TH, Klausner RD: T cell receptor structure and function: Analysis by expression of portions of isolated
subunits, Adv Exper Med Biol 323:1, 1992.
Garcia KC, Degano M, Stanfield RL, et al: An α/β T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex, Science
274(5285):209, 1996.
Garcia KC, Teyton L, Wilson IA, et al: Structural basis of T cell recognition,
Annu Rev Immunol 17:369, 1999.
Gollob JA, Li J, Kawasaki H, et al: Molecular interaction between CD58 and
CD2 counter-receptors mediates the ability of monocytes to augment T
cell activation by IL-12, J Immunol 157(5):1996, 1886.
Gollob JA, Ritz J: CD2-CD58 interaction and the control of T cell internet kin-12 responsiveness. Adhesion molecules link innate and acquired
immunity, Ann NY Acad Sci 795:71, 1996.
Haas W, Pereira P, Tonegawa S, et al: γ/δ cells, Annu Rev Immunol 11:637,
1993.