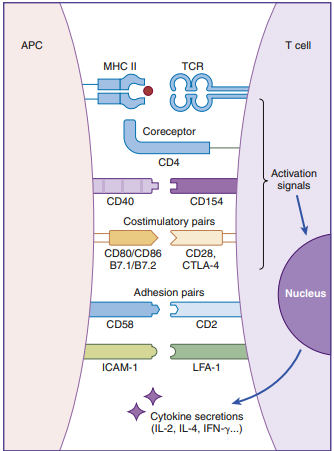
Much like starting an automobile, several signals must be given in a defined sequence to override interlocking safety systems. T cell activation, for example, needs three steps: (1) The lymphocyte T cell receptor (TCR) interacts with the antigen-loaded class II molecule in the first phase. (2) A CD4 molecule attaches to class II molecules in the second phase, stabilizing the TCR–class II complex. (3) The T cell’s CD28 binds with a B7 molecule on the APC in the last stage of the activation cycle (antigen-presenting cell). The T cell becomes nonfunctional for a lengthy period if these three interactions do not occur.
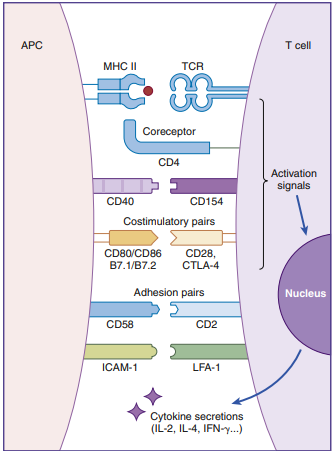
Table of Contents
Clonal selection hypothesis
The clonal selection theory, developed by MacFarland Burnet in the late 1950s, says that antigens, not cells, control the immune response. The five basic theorems are: (1) each lymphocyte has a unique receptor that binds to only one antigen; (2) the receptor must react with the antigen for cellular activation; (3) only selected clones of lymphocytes activated by an antigen will divide (clonal expansion); (4) all daughter cells will express antigen receptors identical to the parent cell; and (5) some of the daughter cells will act as progenitor cells.
α/β T Cell Receptors
The T cell receptor is a heterodimer with two chains, one variable (V) and the other constant (C). The structure of an α/β TCR is shown in the Figure below.
The V regions of the α/β-chains determine antigenic specificity by creating a three-dimensional pocket that recognizes immunogenic epitopes. The dual-chain V region pocket is created through the association of several different genes. The Vα pocket is the result of Vα genes joining with a junctional (Jα) gene. In the β-chain, a functional Vβ is created by linking Vβ, diversity (Dβ), and Jβ-domains. Complementarity determining regions (CDRs), which are hypervariable in the Vα/β-chains have actual contact with antigens.
Class II molecules have six CDRs (three on each chain). The role of CDRs in binding to major histocompatibility complex (MHC) class II molecules is shown in the Figure below. In the α-chains, CDR1 interacts with the N-terminal portion of the epitope. β-chain CDR1 reacts with the C-terminal portion of the peptide. CDR2s interact with the class II molecules but are not involved in antigen recognition. CDR3s are responsible for binding to the epitope presented by class II molecules. Most of the sequence variability responsible for TCR diversity is found in CDR3.
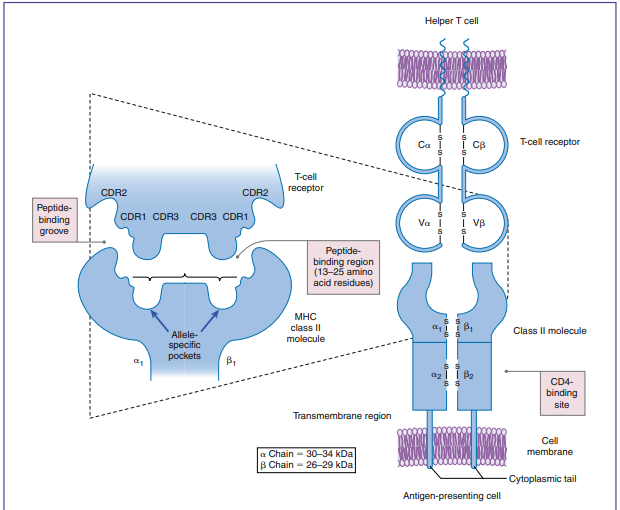
In addition to V regions, each TCR has an invariant constant(C)region. The C region consists of 140 to 180 hydrophilic amino acids and continues to a short hinge region with cysteine residues that link the α-chain and the β-chain A transmembrane anchor unit, which consists of highly charged hydrophobic amino acids, is connected to a short cytoplasmic region.
γ/δ T Cell Receptors
T/ TCRs have a structure similar to / TCRs. Only the mucosa has these T cells, which serve as a link between innate and adaptive immunity. Isopentyl pyrophosphate (IDP) and dimethylallylpyrophosphate (DMAP) are phosphorylated microbial compounds that CD4/T cells detect (DMAP). The T cell is activated by direct contact between these chemicals and the/ TCR. Activation does not need antigen processing by APCs or presentation by MHC molecules. Until an adaptive response is developed, a strong / T cell response localizes the microorganism in the mucosa.
Origin of t cell receptor diversity
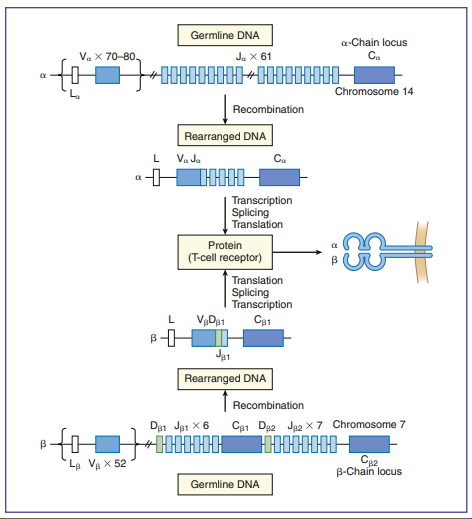
- In the generation of TCR diversity, two factors must be considered.
- First, each lymphocyte has a TCR that recognizes a single epitope.
- Second, 1013 different epitopes must be recognized.
- If a single lymphocyte TCR recognizes only one epitope, it follows that 1013 lymphocyte clones are present in each of the CD4 (Th1 and Th2) and CD8 (Tc1 and Tc2) populations.
- To create a repertoire of antigen-specific TCRs, alternative forms of genes present in somatic cells are rearranged in a process, called somatic cell recombination, by using RAG-1 and RAG-2 recombinase activating enzymes showing in above figure.
- Simple recombination in the α-chain can result in approximately 4.9 × 103 antigen-specific TCRs.
- β-chains have 52 variable gene segments, two diversity gene segments, and 13 J region genes.
- Recombination in the β-chain results in 1.3 × 103 unique TCRs.
- Additional TCR diversity is created by the alternate joining of Dβ-chains, junctional flexibility, and N- and P-nucleotide additions.
- The alternate joining of D domains creates 5 × 103 diverse TCRs.
- Adding six or more amino acids to the J domains (junctional diversity) creates more TCR diversity.
- Random insertion of palindromic sequences at single-strand breaks (P-nucleotides) or nontemplated amino acids (N-nucleotides) into the TCR coding regions also occurs.
- Junctional diversity and P- and N-nucleotides generate an additional 1011 possible TCRs.
- The sum of the gene re-arrangements (4.9 × 103 + 1.3 × 103 + 5.3 × 10 3 + 1.0 × 1011) allows the immune system to respond to all known antigens.