Biological response of the drug molecule are produced when it reach to sufficient concentration at the site of action, and then interact with the body receptor and enzyme.
For better response the drug molecule are fit the receptor site and pass across the various biological membrane or barriers, the physiochemical properties and various stereochemical properties are affect on the transportation of drug molecule from its site of absorption to site of action.
Various physiochemical properties as follows:
IONIZATION:
- For good solubility and absorption of the drug molecule very important to maintain a balance of good ionized and nonionized.
- Ionized form of drug have good aqueous solubility and good binding capacity with the receptor.
- Non-ionized form of drug help to cross the cell membrane.
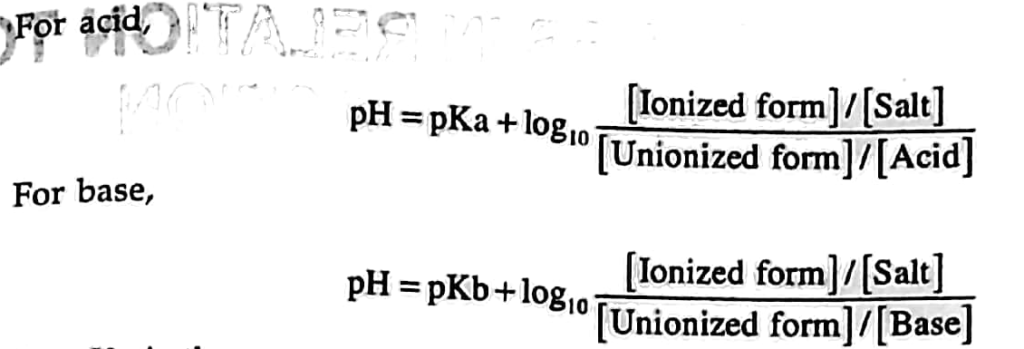
- Drug which are weak acidic or base are absorbed into the gastro-intestinal tract in the form of non-ionized form, the unionized function of both dissociation constant(Pka) and pH of the environment.
- It is represented by the Henderson Hassel batch equation
Drug pKa range between 2 to 4 are readily absorbed by the stomach and the drug pH range more than 8 are absorbed by intestine and acidic drug are absorbed by the stomach.
SOLUBILITY
solubility of the drug in polar solvent(water, acetic acid, methanol, ethanol, n-propanol, n-butanol) and non polar solvent(benzene, carbon tetrachloride, and diethyl ether) depend upon various factor such as chemical structure, particle size of the drug molecule and crystal form.
lipid soluble drug possess high biological activity as compared with water soluble drugs.
PARTITION-COEFFICIENT
- It is defines as the ratio of concentration of a drug molecule in lipid phase and water phase.
- It is determine the transport and diffusion of a drug.
- The diffusion of the drug molecule across the membrane or barrier is depend on the its solubility in lipid i.e on the partition coefficient of the drug.
- More the value of partition coefficient more will be the diffusion across the cell membrane.
HYDROGEN BONDING:
- The hydrogen bond exist in between hydrogen and electronegative atoms like F, Cl, N, O and s.
- It is a relatively weaker bond.
- Hydrogen bond play an important role while the drug attach to the receptor sites, this help in the interaction with specific enzyme and protien.
PROTIEN BINDING:
- In the body drug are interacts with various components like blood and extravascular tissues.
- The binding of drug with protein like macromolecule and blood id two types. 1) Intracellular binding and 2) Extracellular binding
- Protein binding helps in drug Absorption( site of action to systemic circulation), Solubility( water insoluble drugs are drugs are circulated by help by binding of lipoproteins and Distribution( help to distribute drug through body but can’t cross blood brain barriers.
CHELATION:
Chelates are metal-ion complexes that are formed by metal ions and an electron-donating molecule known as ligand. The valence shells of metal ions must be incomplete. Chelation is the term for the method of forming a metal-ligand complex. N, O, and S are some examples of electron atoms. The number of electron donating groups present in a ligand decides the different form of ligand.
- Disodium EDTA form chelates with various toxic metal ions and its help to removal toxic metal ions Ex: poisoning of lead and vanadium.
- Haemoglobin and cyanocobalamine are naturally occuring chelates, Dimercaprol help to treatment of arsenic poisoning.
BIOISOSTERISM:
Bioisosteres are drugs and chemical substituents or functional groups that have the same physical or chemical properties as another chemical compound and produce similar biological properties. The relationship between bioisosteres is known as bioisosterism.
In drug design Bioisosterism plays very importance role. Its help in increases the required biological or physical property of a drug without changing the chemical structure.
Bioisosterism also help to reduced the toxicity which changes or alter the bioavailability of the drug molecule.
OPTICAL AND GEOMETRICAL ISOMERISM:
The stereochemistry of the drug molecule has a significant impact on the drug’s action since it alters the drug’s interaction with specific receptors, transport, biotransformation, and removal. Only one of the stereoisomers has the primary biological activity. Stereoisomers may have similar or dissimilar functions. The three dimensinoal (3D) structure of the drugs molecule gives the detail information the interaction of the drug and receptors and hence drugs activity.