The Difference Between evaporation and other heat processes like Vaporization, Distillation, Drying, and Sublimation…
Table of Contents
Difference between Vaporization and Evaporation
| Vaporization (Boiling) | Evaporation |
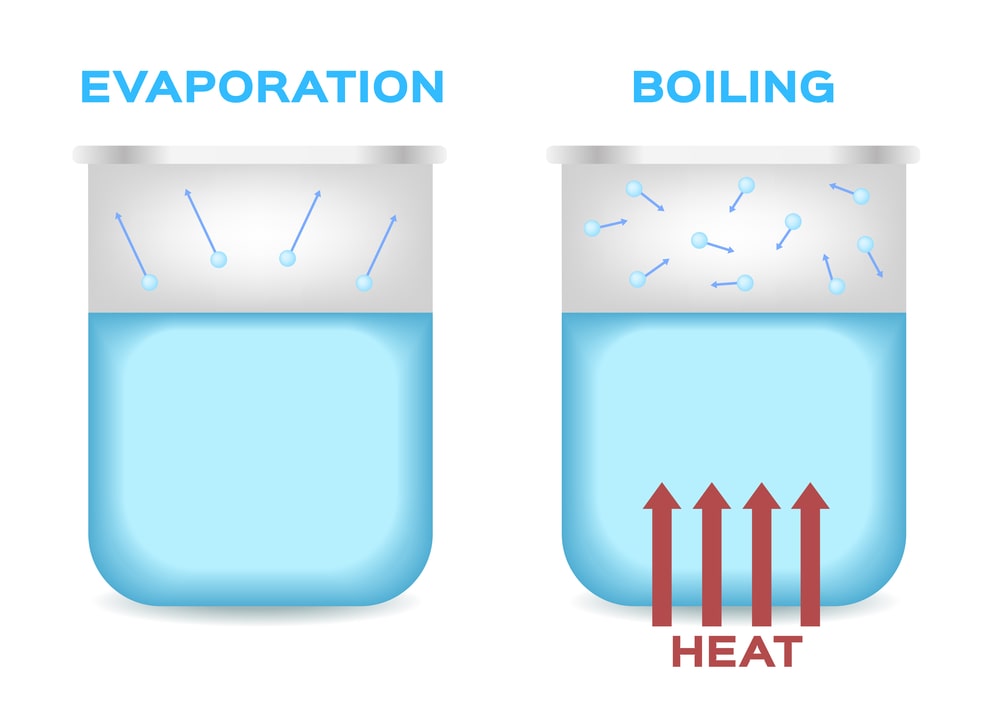
| (i) It is a process in which a substance changes its state from the liquid state to the gaseous state with boiling. | (i) It is a process in which a substance changes its state from the liquid state to the gaseous state without boiling. |
| (ii) It is a fast process. | (ii) It is a slow process. |
| (iii) Bubbles are formed while vaporization. | (iii) No bubbles formed during evaporation. |
| (iv) Occurs throughout the liquid. | (iv) Takes place only from the exposed the surface of the liquid. |
| (v) Vaporization does not depend on the surface area of the liquid. | (v) Evaporation depends on the surface area of the liquid. |
| (vi) It occurs at a definite temperature i.e. at boiling point. | (vi) It occurs at all temperatures. |
| (vii) Heating source of energy is needed. | vii) Energy is supplied by surroundings. |
Difference between Distillation and Evaporation
| Distillation | Evaporation |
| (i) Distillation is a process of separating the component substances from a liquid mixture by selective evaporation and condensation. | (i) Evaporation is a type of vaporization of a liquid that occurs from the surface of a liquid into a gaseous phase. |
| (ii) In the distillation, vaporization takes place at the boiling point | (ii) In evaporation, vaporization takes place below the boiling point. |
| (iii) Distillation is taking place from the whole liquid mass. | (iii) Evaporation takes place only from the the surface of the liquid. |
| (iv) At the boiling point in distillation the liquid forms bubbles. | (iv) No bubble formation in evaporation. |
| (v) Distillation is a separation or purifying technique. | v) Evaporation is not necessarily the separation or purifying technique. |
| (vi) In distillation, heat energy should be supplied to liquid molecules to go into the vapour state. | vi) In evaporation, molecules get energy when they collide with each other and is used to escape to the vapour state. |
| (vii) In distillation, vaporization happens rapidly. | (vii) The evaporation is a slow process. |
| (viii) It involves boiling and condensation. | (viii) It involves only evaporation. |
| (ix) Process of purification and separating components with fractional distillation based on boiling point. | (ix) Process of purification and separating components without fractionation independent of boiling point. |
| (x) Example is water and alcohol mixture at the boiling point of alcohol. | (x) Example is water below boiling point. |
Difference between Drying and Evaporation
| Drying | Evaporation |
| (i) Drying is removal of water from a substance. | (i) Evaporation is the changing of the phase of liquid water to gaseous water. |
| (ii) It is possible to dry a substance without evaporation. For example, using air, adsorbent, absorbent and freeze drying. | (ii) Evaporation is a physical phenomenon that may form part of the mechanisms that effect such a change, but there are others too. |
| (iii) Separation of moisture content from any type of biological, chemical and metallurgical materials due to temperature gradient. | (iii) In evaporation the surface molecules skip to the atmosphere by gaining extra energy, especially in the form of heat. |
| (iv) Drying follows evaporation. | (iv) Evaporation results in drying. |
| (v) Drying is the process of removing moisture from any object. Drying can occur due to evaporation as well as external factors such as wind. | (v) Evaporation is a process in which the surface molecules skip to the atmosphere by gaining extra energy, especially in the form of heat. |
| (vi) Drying is the evaporation or conversion of water molecules to the gaseous state for more stability. | (vi) Evaporation takes place in all liquids content, when left in open, tend to lose its water is usually in atmospheric air. |
| (vii) Drying is the removal of traces of water from material by just sending a hot dry air over it. | (vii) In evaporation mass of liquid at room temperature loses some of the water in it to adjoining environment. |
| (viii) Drying involves the removal of volatile matter by evaporation. In short drying mass transfer takes place by means of the evaporation process. | (viii) Evaporation is the process to concentrate the slurry of solid by the removal of volatile matter. It is a heat transfer operation. |
Difference between Sublimation and Evaporation
| Sublimation | Evaporation |
| (i) Phase change is from solid to gas. | (i) Phase change is from liquid to gas. |
| (ii) No liquid state involves in this process. | (ii) In this liquid is involved. |
| (iii) It involves solid as starting material. | (iii) It involves liquid as starting material. |
| (iv) Requires external energy equal to achieve sublimation. | (iv) Requires external energy equal to achieve evaporation. |
| (v) Example: Camphor, iodine. | (v) Example: Water to vapor. |
Make sure Check our Amazing article on: Factors affecting evaporation