Table of Contents
What is Clinical Pharmacokinetics?
During the drug development process, large numbers of patients are enrolled in clinical trials to determine efficacy and optimum dosing regimens. Along with safety and efficacy data and other patient information, the FDA approves a label that becomes the package insert discussed in more detail later in this chapter. The approved labeling recommends the proper starting dosage regimens for the general patient population and may have additional recommendations for special populations of patients that need an adjusted dosage regimen. These recommended dosage regimens produce the desired pharmacologic response in the majority of the anticipated patient population. However, intra- and interindividual variations will frequently result in either a subtherapeutic (drug concentration below the MEC) or a toxic response (drug concentrations above the minimum toxic concentration, MTC), which may then require adjustment to the dosing regimen. Clinical pharmacokinetics is the application of pharmacokinetic methods to drug therapy inpatient care. Clinical pharmacokinetics involves a multidisciplinary approach to individually optimized dosing strategies based on the patient’s disease state and patient-specific considerations.
The study of the clinical pharmacokinetics of drugs in disease states requires input from medical and pharmaceutical research. Table 1-2 is a list of 10 age-adjusted rates of death from 10 leading causes of death in the United States in 2003. The influence of many diseases on drug disposition is not adequately studied. Age, gender, genetic, and ethnic differences can also result in pharmacokinetic differences that may affect the outcome of drug therapy. The study of pharmacokinetic differences of drugs in various population groups is termed population pharmacokinetic (Sheiner and Ludden, 1992. Application of Pharmacokinetics to Specific Populations will discuss many of the important pharmacokinetic considerations for dosing subjects due to age, weight, gender, renal, and hepatic disease differences. Despite advances in modeling and genetics, sometimes it is necessary to monitor the plasma drug concentration precisely in a patient for safety and multidrug dosing consideration. Clinical pharmacokinetics is also applied to therapeutic drug monitoring (TDM) for very potent drugs, such as those with a narrow therapeutic range, in order to optimize efficacy and to prevent any adverse toxicity. For these drugs, it is necessary to monitor the patient, either by monitoring plasma drug concentrations (eg, theophylline) or by monitoring a specific pharmacodynamic endpoint such as prothrombin clotting time (eg, warfarin). Pharmacokinetic and drug analysis services necessary for safe drug monitoring is generally provided by the clinical pharmacokinetic service (CPKS). Some drugs frequently monitored are aminoglycosides and anticonvulsants. Other drugs closely monitored are those used in cancer chemotherapy, in order to minimize adverse side effects (Rodman and Evans, 1991).
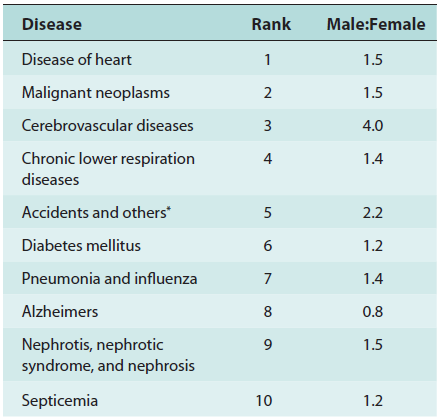
Rates, by Male/Female Ratio from the
10 Leading Causes of Death* in the US, 2003
Labeling For Human Prescription Drug and Biological Products
In 2013, the FDA redesigned the format of the prescribing information necessary for safe and effective use of the drugs and biological products (FDA Guidance for Industry, 2013). This design was developed to make information in prescription drug labeling easier for health care practitioners to access and read. The practitioner can use the prescribing information to make prescribing decisions. The labeling includes three sections:
• Highlights of Prescribing Information (Highlights)—contains selected information from the Full Prescribing Information (FPI) that health care practitioners most commonly reference and consider most important. In addition, highlights may contain any boxed warnings that give a concise summary of all of the risks described in the Boxed Warning section in the FPI.
• Table of Contents (Contents)—lists the sections and subsections of the FPI.
• Full Prescribing Information (FPI)—contains the detailed prescribing information necessary for safe and effective use of the drug.
An example of the Highlights of Prescribing Information and Table of Contents for Nexium (esomeprazole magnesium) delayed-release capsules appears in Table 1-3B. The prescribing information sometimes referred to as the approved label or the package insert may be found at the FDA website, Drugs@FDA (http://www.accessdata.fda.gov/scripts/cder/drugsatfda/). Prescribing information is updated periodically as new information becomes available. The prescribing information contained in the label recommends dosage regimens for the average patient from data obtained from clinical trials. The indications and usage section are those indications that the FDA has approved and that have been shown to be effective in clinical trials. On occasion, a practitioner may want to prescribe the drug to a patient drug for a non-approved use or indication. The pharmacist must decide if there is sufficient evidence for dispensing the drug for a non-approved use (off-label indication). The decision to dispense a drug for a non-approved indication may be difficult and often made with the consultation of other health professionals.
Clinical Pharmacology
Pharmacology is a science that generally deals with the study of drugs, including their mechanism, effects, and uses of drugs; broadly speaking, it includes pharmacognosy, pharmacokinetics, pharmacodynamics, pharmacotherapeutics, and toxicology. The application of pharmacology in clinical medicine including clinical trial is referred to as clinical pharmacology. For pharmacists and health professionals, it is important to know that NDA drug labels report many important study information under Clinical Pharmacology in Section 12 of the standard prescription label (Tables 1-3A and 1-3B).
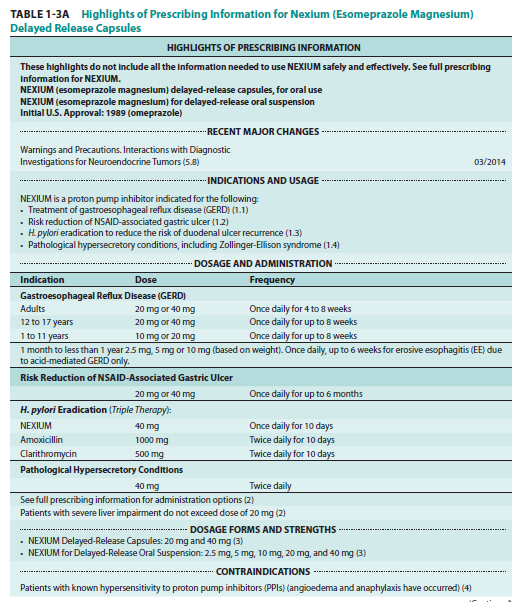
Delayed-Release Capsules, Clinical Pharmacokinetics
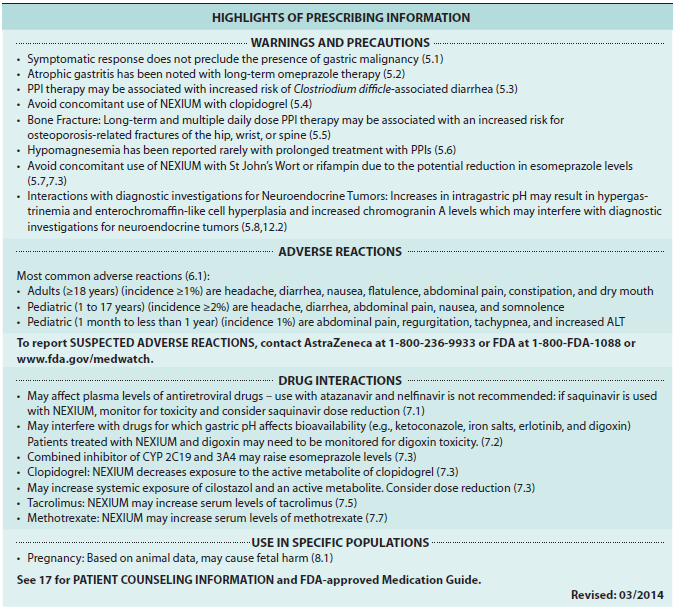
Delayed-Release Capsules (Continued), Clinical Pharmacokinetics
Pharmacogenetics
Pharmacogenetics is the study of drug effects including distribution and disposition due to genetic differences, which can affect individual responses to drugs, both in terms of therapeutic effect and adverse effects. A related field is pharmacogenomics, which emphasizes different aspects of genetic effect on drug response. Pharmacogenetics is the main reason why many new drugs still have to be further studied after regulatory approval, that is, the postapproval phase 4 studies. The clinical trials prior to drug approval are generally limited such that some side effects and special responses due to genetic differences may not be adequately known and labeled.