Table of Contents
Drug product performance
Drugs are compounds that are meant to be used in the diagnosis, treatment, mitigation, or prevention of disease. For systemic or local therapeutic activity, drugs are delivered in a range of dosage forms or drug products, such as solids (tablets, capsules), semisolids (ointments, creams), liquids, suspensions, emulsions, and so on. Drug products are medication delivery systems that release and distribute the drug to the site of action in order to achieve the intended therapeutic effect. Furthermore, drug items are created with the patient’s demands in mind, such as palatability, convenience, and safety.
The release of the drug substance from the drug product, either for local drug action or for drug absorption into the plasma for systemic therapeutic activity, is referred to as drug product performance. Pharmaceutical technology and manufacturing advancements have centered on creating high-quality drug products that are safer, more effective, and more convenient for patients.
Biopharmaceutics
The effects of the physical/chemical qualities of the medication, the dosage form (drug product) in which the drug is delivered, and the route of administration on the rate and extent of systemic drug absorption are studied in biopharmaceutics. The impact of the drug substance and formulation on absorption and in vivo distribution of the drug to the site of action is described as a series of events that occur before the therapeutic effect of the drug is elicited. A general scheme describing this dynamic relationship is
illustrated in Fig. 1-1.
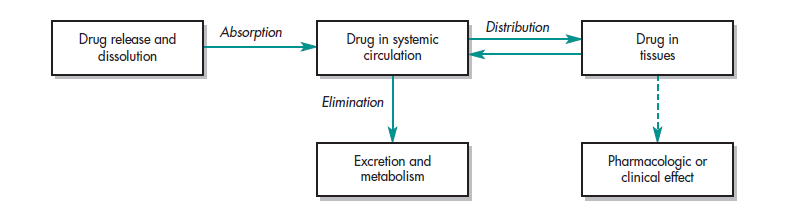
In the study of biopharmaceutics First, the drug in its dosage form is taken by the patient by an oral, intravenous, subcutaneous, transdermal, etc, route of administration. Next, the drug is released from the dosage form in a predictable and characterizable manner. Then, some fraction of the drug is absorbed from the site of administration into either the surrounding tissue for local action or into the body (as with oral dosage forms) or both. Finally, the drug reaches the site of action. A pharmacodynamic response results when the drug concentration at the site of the action reaches or exceeds the minimum effective concentration (MEC). The suggested dosing regimen, including starting dose, maintenance dose, dosage form, and dosing interval, is determined in clinical trials to provide the drug concentrations that are therapeutically effective in most patients. This sequence of events is profoundly affected—in fact, sometimes orchestrated—by the design of the dosage form and the physicochemical properties of the drug.
Historically, pharmaceutical scientists have evaluated the relative drug availability to the body in vivo after giving a drug product by different routes to an animal or human, and then comparing specific pharmacologic, clinical, or possible toxic responses. For example, a drug such as isoproterenol causes an increase in heart rate when given intravenously but has no observable effect on the heart when given orally at the same dose level. In addition, the bioavailability (a measure of systemic availability of a drug) may differ from one drug product to another containing the same drug, even for the same route of administration. This difference in drug bioavailability may be manifested by observing the difference in the therapeutic effectiveness of the drug products. Thus, the nature of the drug molecule, the route of delivery, and the formulation of the dosage form can determine whether an administered drug is therapeutically effective is toxic, or has no apparent effect at all.
The US Food and Drug Administration (FDA) approves all drug products to be marketed in the United States. The pharmaceutical manufacturers must perform extensive research and development prior to approval. The manufacturer of a new drug product must submit a New Drug Application (NDA) to the FDA, whereas a generic drug pharmaceutical manufacturer must submit an Abbreviated New Drug Application (ANDA). Both the new and generic drug product manufacturers must characterize their drug and drug products and demonstrate that the drug product performs appropriately before the products can become available to consumers in the United States.
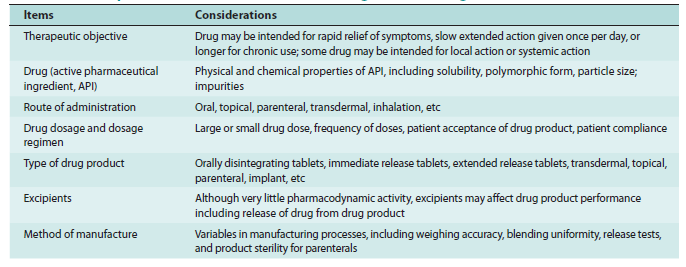
Biopharmaceutics provides the scientific basis for drug product design and drug product development. Each step in the manufacturing process of a finished dosage form may potentially affect the release of the drug from the drug product and the availability of the drug at the site of action. The most important steps in the manufacturing process are termed critical manufacturing variables. Examples of biopharmaceutical considerations in drug product design are listed inTable 1-1. It is important for a pharmacist to know that drug product selection from multi-sources could be confusing and needs a deep understanding of the testing procedures and manufacturing technology that is included in the chemistry, manufacturing, and control (CMC) of the product involved. The starting material (SM) used to make the API (active pharmaceutical ingredient), the processing method used during chemical synthesis, extraction, and the purification method can result in differences in the API that can then affect drug product performance. Sometimes a by-product of the synthetic process, residual solvents, or impurities that remain may be harmful or may affect the product’s physical or chemical stability. Increasingly, many drug sources are imported and the manufacturing of these products is regulated by codes or pharmacopeia in other countries. For example, drugs in Europe may be meeting EP (European Pharmacopeia), and since 2006, agreed uniform standards are harmonized in ICH guidances for Europe, Japan, and the United States. In the US, the USP-NF is the official compendia for drug quality standards.
Finally, the equipment used during manufacturing, processing, and packaging may alter important product attributes. Despite compliance with testing and regulatory guidance involved, the issues involving pharmaceutical equivalence, bioavailability, bioequivalence, and therapeutic equivalence often evolved by necessity. The implications are important regarding the availability of quality drug products, avoidance of shortages, and maintaining an affordable high-quality drug products.
Thus, biopharmaceutics involves factors that influence
- The design of the drug product
- stability of the drug within the drug product,
- the manufacture of the drug product,
- the release of the drug from the drug product,
- the rate of dissolution/release of the drug at the absorption site, and
- delivery of drug to the site of action,
which may involve targeting the drug to a localized area (eg, colon for Crohn disease) for action or for systemic absorption of the drug.
Both the pharmacist and the pharmaceutical scientist must understand these complex relationships to objectively choose the most appropriate drug product for therapeutic success. The study of biopharmaceutics is based on fundamental scientific principles and experimental methodology. Studies in biopharmaceutics use both in vitro and in vivo methods. In vitro methods are procedures employing test apparatus and equipment without involving laboratory animals or humans. In vivo methods are more complex studies involving human subjects or laboratory animals. These methods must be able to assess the impact of the physical and chemical properties of the drug, drug stability, and large-scale production of the drug and drug product on the biological performance of the drug. analytical methods for the measurement of drugs and metabolites, and procedures that facilitate data collection and manipulation. The theoretical aspect of pharmacokinetics involves the development of pharmacokinetic models that predict drug disposition after drug administration. The application of statistics is an integral part of pharmacokinetic studies. Statistical methods are used for pharmacokinetic parameter estimation and data interpretation ultimately for the purpose of designing and predicting optimal dosing regimens for individuals or groups of patients. Statistical methods are applied to pharmacokinetic models to determine data error and structural model deviations. Mathematics and computer techniques form the theoretical basis of many pharmacokinetic methods. Classical pharmacokinetics is a study of theoretical models focusing mostly on model development and parameterization.
Pharmacokinetics
After a drug is released from its dosage form, the drug is absorbed into the surrounding tissue, the body, or both. The distribution through and elimination of the drug in the body varies for each patient but can be characterized using mathematical models and statistics. Pharmacokinetics is the science of the kinetics of drug absorption, distribution, and elimination (ie, metabolism and excretion). The description of drug distribution and elimination is often termed drug disposition. Characterization of drug disposition is an important prerequisite for the determination or modification of dosing regimens for individuals and groups of patients.
The study of pharmacokinetics involves both experimental and theoretical approaches. The experimental aspect of pharmacokinetics involves the development of biologic sampling techniques, analytical methods for the measurement of drugs and metabolites, and procedures that facilitate data collection and manipulation. The theoretical aspect of pharmacokinetics involves the development of pharmacokinetic models that predict drug dispositions after drug administration. The application of statistics is an integral part of pharmacokinetic studies. Statistical methods are used for pharmacokinetic parameter estimation and data interpretation ultimately for the purpose of designing and predicting optimal dosing regimens for individuals or groups of patients. Statistical methods are applied to pharmacokinetic models to determine data error and structural model deviations. Mathematics and computer techniques form the theoretical basis of many pharmacokinetic methods. Classical pharmacokinetics is a study of theoretical models focusing mostly on model development and parameterization.
Pharmacodynamics
Pharmacodynamics is the study of the biochemical and physiological effects of drugs on the body; this includes the mechanisms of drug action and the relationship between drug concentration and effect. A typical example of pharmacodynamics is how a drug interacts quantitatively with a drug-receptor to produce a response (effect). Receptors are the molecules that interact with specific drugs to produce a pharmacological effect in the body. The pharmacodynamic effect sometimes referred to as the pharmacologic effect, can be therapeutic and/or cause toxicity. Often drugs have multiple effects including the desired therapeutic response as well as unwanted side effects. For many drugs, the pharmacodynamic effect is dose/drug concentration-related; the higher the dose, the higher drug concentrations in the body, and the more intense the pharmacodynamic effect up to a maximum effect. It is desirable that side effects and/or toxicity of drugs occur at higher drug concentrations than the drug concentrations needed for the therapeutic effect. Unfortunately, unwanted side effects often occur concurrently with the therapeutic doses.
Make sure also check the article on: Biochemistry and Medicine