Quantum theory of radiation: The wave hypothesis of radiant energy transmission seemed to indicate that energy was released (or absorbed) in waves. Max Planck investigated the spectral lines produced from hot-body radiations at various temperatures in 1900. According to him, light radiation was created intermittently by the heated body’s molecules, each of which vibrated at a particular frequency that grew with temperature. As a result, Planck presented a new hypothesis in which a heated substance emits energy in tiny units of waves rather than continuous waves. Quantum refers to the ‘unit wave’ or ‘energy pulse’ (plural, quanta).
The general Quantum Theory of Electromagnetic Radiation in its present form may be stated as :
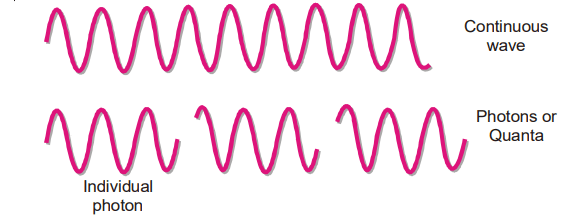
(1) When atoms or molecules absorb or emit radiant energy, they do so in separate ‘units of waves’ called quanta or photons. Thus light radiations obtained from energized or ‘excited atoms’ consist of a stream of photons and not continuous waves.
(2) The energy, E, of a quantum or photon is given by the relation E = hν ……..(1)
where ν is the frequency of the emitted radiation, and h the Planck’s Constant. The value of h = 6.62 × 10-27 erg sec. or 6.62 × 10-34 J sec. We know that c, the velocity of radiation, is given by the equation c = λν……(2)
Substituting the value of ν from (2) in (1), we can write E=hc/ λ
As a result, the size of a quantum or photon of energy is proportional to the radiant energy’s frequency, or inversely proportional to its wavelength.
(3) An atom or molecule can emit (or absorb) either one quantum of energy (hν) or any whole-number multiple of this unit.
As a result, radiant energy can be emitted as hv, 2h, 3h, and so on, but never as 1.5 h, 3.27 h, 5.9 h, or any other fractional value of h, such as nh.
Quantum theory served as an excellent foundation for describing the photoelectric phenomenon, atomic spectra, and current ideas of atomic and molecule structure.