Benzene has 6 hydrogen which all are equal. If they replaced anyone hydrogen (H) atom by substituent it gives a mono substituent derivative of benzene. If they replace 2 or 3 substituents, then it’s give a di and poly substituted derivative.
when mono substituent benzene is converted into di-substituted in derivative, then three isomers (it’s known as ortho, meta and para) are possible.
The substituents which is already present in the ring determined the position of the incoming group. The ability of a group already present in the ring to direct the incoming group is called as directive influence of group. The group which are already present in the Benzene its not only decide the orientation of further substitution but also affect the reactivity of the benzene nucleus towards further electrophilic substitution reaction.
EFFECT OF SUBSTITUENTS ON Orientation: A second substituents can occupy any of the remaining five position in the monosubstituted Benzene because of 1 substituted which are already present in the Benzene.
The position 2 and 6 are equivalent and gives ortho product, whereas position 3 and 5 are equivalent and it’s give meta product. Position 4 is unique and its give para product. There are two ortho, two meta and one para substitution with respect to substituents which is already present.
On the basis of directive influence of groups, all the groups can be divided into following two classes:
A) Ortho-para directing groups: These groups direct the incoming groups to the ortho and para positions. For example, Alkyl(R), phenyl(-C6H6), Halogen (-cl, -Br F I), hydroxyl(-OH), amino(-NH2) etc.
B) Meta directing groups: this groups direct the incoming groups to the meta position. For example: trialkyl ammonium ion (N+R3), nitro(NO2), Cyano(-CN), aldehyde (-CHO), Carboxylic (-COOH). etc.
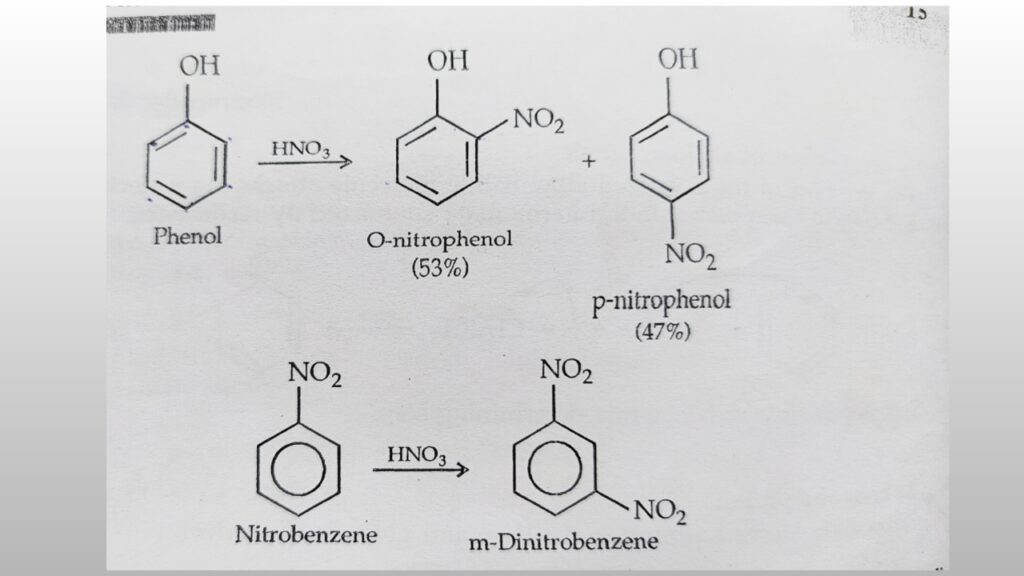
in short, substituent which contain multiple bonds its may double or triple, are usually meta directing and which do not contain any multiple bonds but contain one or more pairs of electrons on the atom are ortho and para directing.
effect of substituents on reactivity: ortho and para directing groups (except alkyl and phenyl) contains one or more pair of electrons on the atom this electrons interact with the π-electrons of the Benzene and increase the electron density. Hence benzene ring gets activated for further electrophilic substitution. So all the ortho and para directing groups are activating groups accept halogens.
meta directing groups due to the presence of multiple bonds in that where withdraw electrons from the benzene ring and it’s decrease the electron density of benzene and deactivates the benzene ring for further electrophilic substitution. Meta directing groups are called as deactivating groups because of it activates the Benzene.
A substance which activates the benzene ring for further electrophilic substitution is called as activating substituent whereas a substance which deactivates a benzene ring for further substitution is known as deactivating substrate.