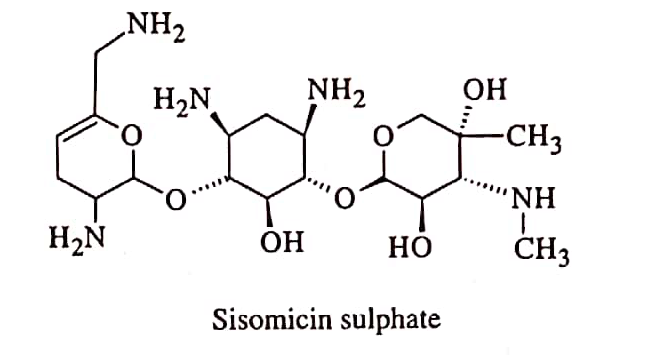
sar of aminoglycosides
Aminoglycosides antibiotics have two distinct structural characteristics. They are the amino sugar part and the hexose ring in the center, which can be 2-deoxystreptamine or streptidine.
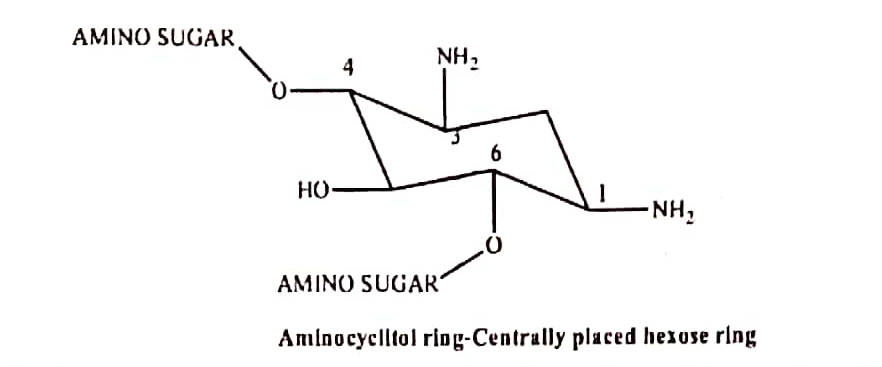
- In this antibiotic contains two or more amino sugars joined in glycoside linkage and a centrally located substituted 1,3-diaminocyclo hexane (aminocyclitol) loop.
- Except for streptomycin, where the ring is streptidine, all aminoglycosides have a 2-deoxy streptamine ring. Two amino sugars are bound to strepidine in streptomycine.
- Two amino sugars are bound to 2-deoxy strepamine in the kanamycin and gentamycin families.


- Amino sugars are bound to 2-deoxy streptamine in the neomycin family.
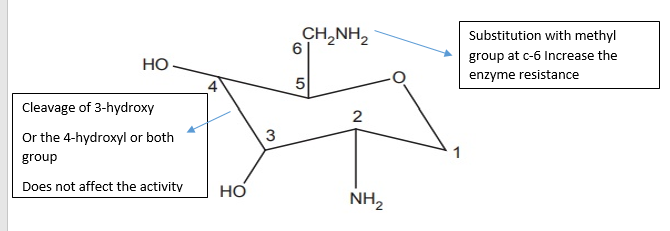
- The C-6 and C-2 positions are targeted by bacterial inactivating enzymes, and the substitution of a methyl group at C-6 increases enzyme resistance.
- The activity is unaffected by cleavage of the 3-hydroxyl, 4-hydroxyl, or both groups.
- While acylation (for example, amikacin) and ethylation (for example, 1-N-ethylsisomycin) do not increase activity, they do help to maintain antibacterial potency.
- Substitution on position 2 (2-hydroxylation) and substitution on position 5 (5-deoxygenation) increase bacterial inactivating enzyme system inhibition. (Derivatives of sisomicin)