Table of Contents
What is Stereoisomerism
Stereoisomerism: Stereochemistry helps to define the structure of a molecule and the orientation of the atoms and functional groups present, in three dimensions. Stereoisomers possess the same molecular and structural formulae and the same functional groups but differ in the three-dimensional spatial orientation of these atoms or groups within the molecule. Due to the difference in orientation of the functional group and the geometry of the molecule, stereoisomers differ in their physical, chemical, physicochemical, and biochemical properties. Based on symmetry and energy criteria, stereoisomers are divided into three classes.
Types of Stereoisomerism
(a) Geometrical isomers
(b) Optical isomers
(c) Conformational isomers.
(a) Geometrical isomers (cis-trans isomerism)
Maleic acid (m.p. 130°C) and fumaric acid (m.p. 287°C) have the same molecular formula but differ in the arrangement of functional groups around the double bond. They have different physical and, to some extent, chemical properties. This type of isomerism is known as geometrical isomerism.
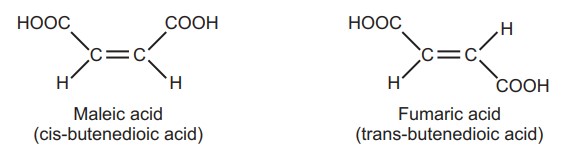
The presence of a carbon-carbon double bond restricts the freedom of rotation about the double bond. The designation cis (Latin word: same side), is used to denote the presence of like atoms or groups on the same side and trans (Latin word, across) is used when they are on opposite sides. Isomerism seen in non-cyclic, open-chain compound due to the presence of a double bond, is called π diastereoisomerism while when it occurs in a cyclic skeleton lacking a double bond, it is termed as σ diastereoisomerism.
(b) Optical Isomerism (enantiomerism)
In 1815, Biot found that a number of organic and inorganic compounds in the solution form, have the ability to rotate the plane of polarized light in opposite directions but in identical amplitude, passing through them. Optical isomerism is seen in compounds that can rotate plane polarised light. A carbon atom connected to four chemically different functional groups is known as asymmetric or chiral carbon and the presence of at least one asymmetric carbon atom in the structure is the pre-requirement for a molecule to show optical isomerism.
If there is one asymmetric carbon then two optically active isomers are possible. Isomer rotating plane of polarized light to the right is said to be dextrorotatory (Latin, dexter: right) while isomer showing rotation to the left is known as laevorotatory (Latin, laevus : left). Both isomers are mirror images of each other yet are not superimposable. They are called enantiomers and the pair of enantiomers is called as enantiomorph. An enantiomer does not possess a plane or center of symmetry. For example,
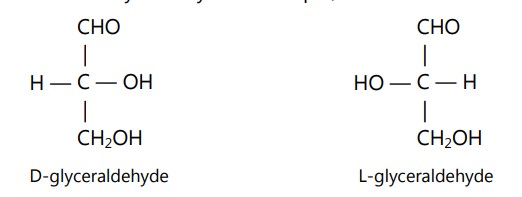
When the enantiomers are present together in equal concentration, the rotation of plane-polarized light caused by laevo isomer will be neutralized by a dextro rotating isomer and the mixture will be optically inactive. Such mixtures are called as racemic mixtures. The conversion of an enantiomer into a racemic form is called as racemization. While the separation of a racemic mixture into individual enantiomers is called as resolution. The maximum number of optically active isomers possible for a molecule having more than one asymmetric carbon atom may be given by the formula
N = 2n
where,
N = Number of optically active isomers,
and n = Number of asymmetric carbon atoms.
With the exception of rotation of plane-polarized light, enantiomers have identical physical and chemical properties like boiling point, melting point, solubility. Their chemical properties are the same towards achiral reagents, solvents, and conditions. Towards chiral reagents, solvents, and catalysts, enantiomers react at different rates.
As per the rule given above, tartaric acid will have four optically active forms because of the presence of two asymmetric carbon atoms
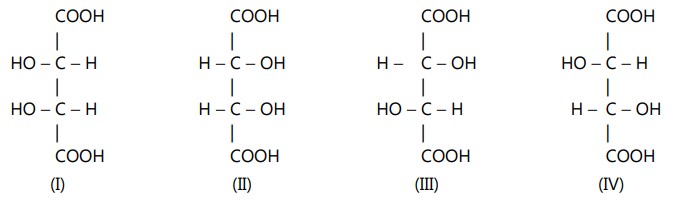
Forms (I) and (II) are identical and symmetrical. In these forms, the upper half is the mirror image of the lower half. This makes the molecule optically inactive through internal compensation. Such identical and symmetrical stereoisomers are called meso-isomers.
Forms (III) and (IV) are mirror images of each other but are not superimposable. They are enantiomeric forms.
While if you compare (III) with (I) or (IV) with (I), these are not enantiomeric pairs. They are neither mirror images nor superimposable. Only one of the two halves of their molecules is identical while the remaining halves are mirror images. Such stereoisomers which do not mirror images and are non-superimposable are called diastereomers. They have different physical and chemical properties, with both achiral and chiral reagents. The rates are different and the product may be different.
Importance of optical isomerism
Rather than racemic mixes, nearly all naturally occurring compounds with asymmetric carbon atoms are in the d or l form. The majority of undesirable effects and poor potency in medications and pharmaceuticals can be attributed to the use of the substance in its racemic combination. Because enantiomers are more active and selective in their pure form, there is a growing desire to commercialise drugs in their active enantiomeric form rather than their racemic form. Optical isomerism has also been used to deduce the mechanism of a variety of chemical processes.
The enantiomer that rotates a beam of polarised light in the clockwise direction is indicated by the prefix (+), formerly d (+) or dextro (–), the other enantiomer rotates light in a counter-clockwise direction and is indicated by the prefix (–), formerly l(–) or Levo.
They have identical chemical and physical properties in an achiral environment but form different products when reacted with other chiral molecules and exhibit optical activity.
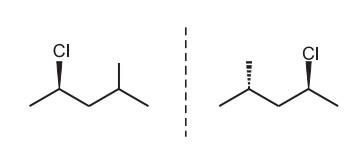
Diasterioisomerism
Stereoisomers with two or more asymmetric or chiral carbons (stereocenter) will show diasterioisomerism. The stereoisomers that are neither mirror images of one another nor are superimposable are known as Diasterioisomerism.
For example:
Also read: Morphology of cell injury adaptive changes