Aminoglycosides antibiotics
Aminoglycosides are broad-spectrum antibiotics, their greater usefulness lies in the treatment of serious systemic infection caused by aerobic gram-negative bacilli.
SAR of aminoglycosides antibiotics (structure-activity relationship) of aminoglycosides antibiotics, Amine group plays a key role in aminoglycosides which can have variable effects when it is attached to any of three rings.
SAR of aminoglycosides antibiotics
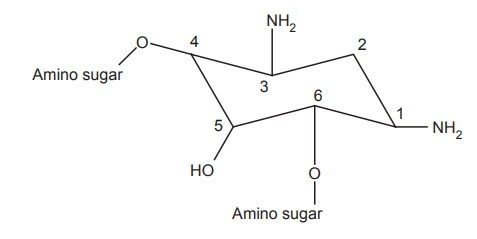
Two structural properties distinguish aminoglycoside antibiotics. The amino sugar part and the centrally located hexose ring, which is either 2-deoxystreptamine or streptidine, are the two components.
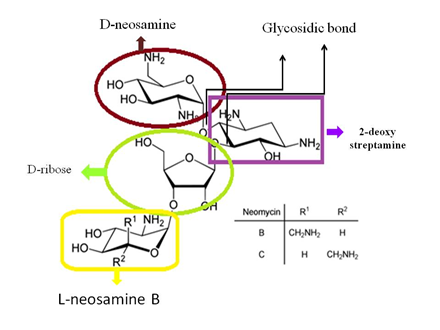
- A centrally located substituted 1, 3- diaminocyclo hexane(aminocyclitol) ring and two or more amino sugar linked in glycosides linkage in aminoglycosides.
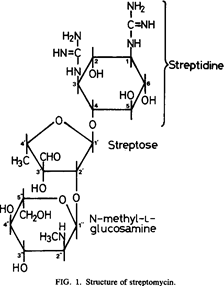
- Except for streptomycin, where the ring is streptidine, all aminoglycosides have a 2-deoxy streptamine ring. Streptidine is connected to two amino sugars in streptomycin.
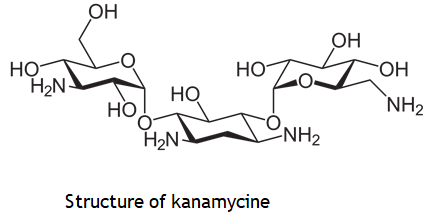
3. Two amino sugars are linked to 2-deoxy streptamine in kanamycin and gentamycin families.
4.Not only kanamycin and gentamycin, but Amino sugars are also linked to 2-deoxy streptamine in the neomycin family.
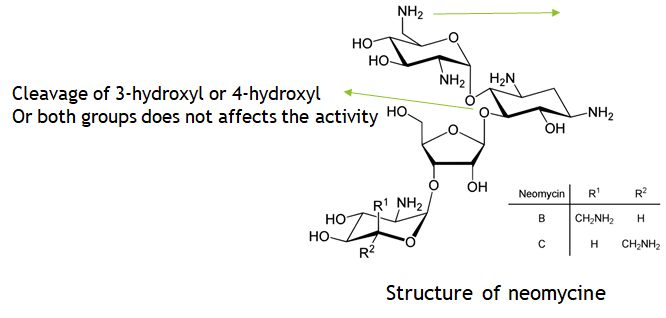
- The C-6 and C-2 locations are targeted by bacterial inactivating enzymes, and substituting a methyl group at C-6 enhances enzyme resistance.
- furthermore Cleavage of the 3-hydroxyl or 4-hydroxyl groups, or both, has no effect on activity.
- Although acylation (e.g. amikacin) and ethylation (e.g. 1-N-ethylsisomycin) do not increase activity, they do aid to maintain antibacterial effectiveness.
- similarly, Substitution on position 2 (2-hydroxylation) and substitution on position 5 (5-deoxygenation) improves bacterial inactivating enzyme system inhibition.
