Membrane transport is essential for cellular life. As cells proceed through their life cycle, a vast amount of exchange is necessary to maintain function. Transport may involve the incorporation of biological molecules and the discharge of waste products that are necessary for normal function.
Table of Contents
Pharmacokinetics
The quantitative study of drug transport in, through, and out of the body is known as pharmacokinetics. The intensity of the response is related to the concentration of the drug at the site of action, which in turn is dependent on its pharmacokinetic properties. Pharmacokinetic considerations, therefore, determine the route (s) of administration, dose, the latency of onset, time of peak action, duration of action, and frequency of administration of a drug.
Membrane Transport
All pharmacokinetic processes involve the transport of the drug across biological membranes.
Biological membrane
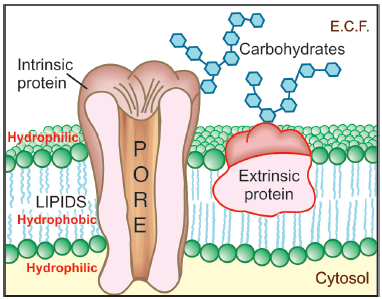
membrane (Membrane Transport)
- This is a bilayer (about 100 Å thick) of phospholipid and cholesterol molecules, the polar groups (glyceryl phosphate attached to ethanolamine/choline or hydroxyl group of cholesterol) of these are oriented at the two surfaces and the nonpolar hydrocarbon chains are embedded in the matrix to form a continuous sheet.
- This imparts high electrical resistance and relative impermeability to the membrane.
- Extrinsic and intrinsic protein molecules are adsorbed on the lipid bilayer.
- Glycoproteins or glycolipids are formed on the surface by attachment to polymeric sugars, aminosugars or sialic acids.
- The specific lipid and protein composition of different membranes differs according to the cell or the organelle type.
- The proteins are able to freely float through the membrane: associate and organize or vice versa.
- Some of the intrinsic ones, which extend through the full thickness of the membrane, surround fine aqueous pores.
- Paracellular spaces or channels also exist between certain epithelial/endothelial cells.
- Other adsorbed proteins have enzymatic, carrier, receptor or signal transduction properties.
- Lipid molecules also are capable of lateral movement.nThus, biological membranes are highly dynamic structures.
Drugs are transported across the membranes by: (a) Passive diffusion and filtration (b) Specialized transport
Passive diffusion
The medication diffuses through the membrane in the direction of its concentration gradient, with the membrane acting as a passive facilitator. For the vast majority of medicines, this is the most significant mechanism; pharmaceuticals are alien molecules (xenobiotics), and the body develops specialised mechanisms largely for natural metabolites.
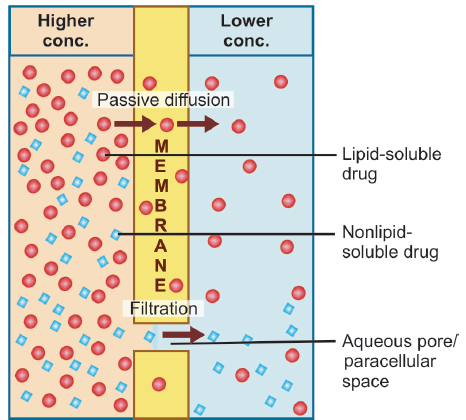
across the lipoidal biological membrane with aqueous
pores (Membrane Transport)
Lipid soluble drugs diffuse by dissolving in the lipoidal matrix of the membrane, the rate of transport being proportional to the lipid : water partition coefficient of the drug. A more lipid-soluble drug attains higher concentration in the membrane and diffuses quickly. Also, greater the difference in the concentration of the drug on the two sides of the membrane, faster is its diffusion.
Influence of pH
Most drugs are weak electrolytes, i.e. their ionization is pH dependent (contrast strong electrolytes that are nearly completely ionized at acidic as well as alkaline pH). The ionization of a weak acid HA is given by the equation:
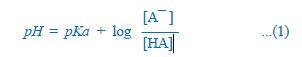
pKa is the negative logarithm of acidic dissociation constant of the weak electrolyte. If the concentration of ionized drug [A¯ ] is equal to concentration of unionized drug [HA], then
[A¯ ]/[HA]=1
since log 1 is 0, under this condition
pH = pKa …(2)
Thus, pKa is numerically equal to the pH at which the drug is 50% ionized.
If pH is increased by 1 scale, then—
log [A¯ ]/[HA] = 1 or [A¯ ]/[HA] = 10
Similarly, if pH is reduced by 1 scale, then—
[A¯ ]/[HA] = 1/10
Thus, weakly acidic drugs, which form saltswith cations, e.g. sod. phenobarbitone, sod. sulfadiazine, pot. penicillin-V, etc. ionize more at alkaline pH and 1 scale change in pH causes 10 fold change in ionization.
Strongly basic drugs, e.g. atropine sulfate, ephedrine HCl, chloroquine phosphate, etc. conversely ionize more at acidic pH. Ions being lipid insoluble, do not diffuse and a pH difference across a membrane can cause differential distribution of weakly acidic and weakly basic drug anions on the two sides. This is because they form salts with anions, which form salts that are insoluble in fatty acids.
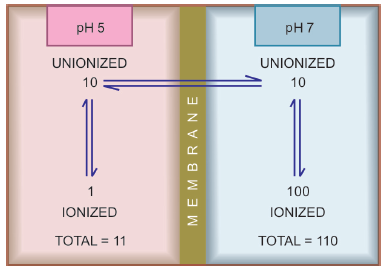
biological membrane on the steady-state distribution of
a weakly acidic drug
Implications of this consideration are:
A) Drugs, e.g. aspirin (pKa 3.5) are largely unionized at acid gastric pH and are absorbed from stomach, while bases, atropine (pka 10) are ionized and are taken only when they reach the intestines.
B) The unionized form of acidic drugs which crosses the surface membrane of gastric mucosal cell, reverts to the ionized form within the cell and then only slowly passes to the extracellular fluid. This is called ion trapping, i.e. a weak electrolyte crossing a membrane to encounter a pH from which it is not able to escape easily.
(C) Basic drugs attain higher concentration intracellularly (pH 7.0 vs 7.4 of plasma).
D) Drugs excreted faster if urine is acidified than if it is alkaline, so they do not back diffuse in the kidney tubules and are then released faster.
Filtration
Filtration is passage of drugs through aqueous pores in the membrane or through paracellular spaces. This can be accelerated if hydrodynamic flow of the solvent is occurring under hydrostatic or osmotic pressure gradient, e.g. across most capillaries including glomeruli. Lipid-insoluble drugs cross biological membranes by filtration if their molecular size is smaller than the diameter of the pores. Most cells have very small pores (4 Å) and drugs with MW > 100 or 200 are not able to penetrate.
Specialized transport
This can be carrier mediated or by pinocytosis.
Carrier transport
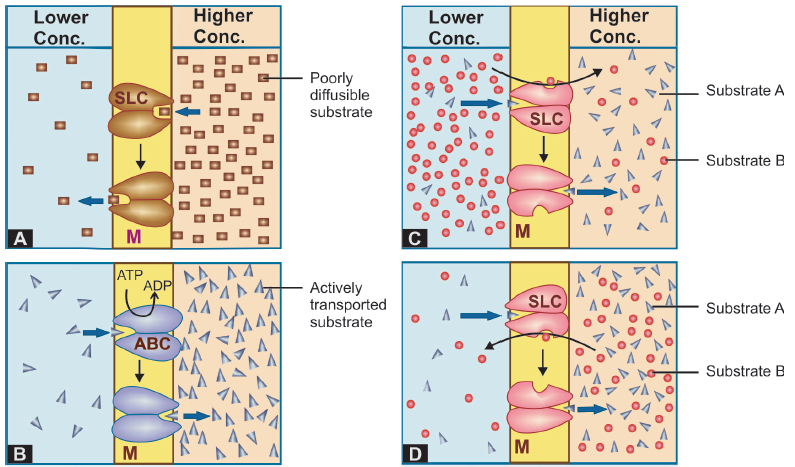
All cell membranes express a host of transmembrane proteins which serve as carriers or transporters for physiologically important ions, nutrients, metabolites, transmitters, etc. across the membrane. At some sites, certain transporter translators also translocate xenobiotics, including drugs and their metabolites. Transporter transport is specific for the substrate (or the type of substrate, e.g. an organic anion), competitively inhibited by analogues which utilize the same transporter, and slower than the flux through channels.
Depending on requirement of energy, carrier transport
rt is of two types:
A. Facilitated diffusion:
The transporter, belonging to the super-family of solute carrier (SLC) transporters, operates passively without needing energy and translocates the substrate in the direction of its electrochemical gradient, i.e. from higher to lower concentration.
B. Active transport:
It requires energy, is inhibited by metabolic poisons, and transports the solute against its electrochemical gradient (low to high), resulting in selective accumulation of the substance on one side of the membrane.
Primary active transport Energy is obtained directly by the hydrolysis of ATP. The transporters belong to the superfamily of ATP binding cassettee (ABC) transporters whose intracellular loops have ATPase activity.
Secondary active transport In this type of active transport effected by another set of SLC transporters, the energy to pump one solute is derived from the downhill movement of another solute (mostly Na+).
Make sure check out our other amazing articles: What are various routes of drug administration