In quantitative term the solubility is defined as ” At a specific temperature, maximum amount of solute that can be dissolved in an amount of solvent.”
In qualitative term solubility can be defined as when salute (it may be solid liquid or gaseous) is to be dissolve in solvent (the solvent may be solid liquid or gaseous) it form a homogeneous solution of the solute in the solvent.
IUPAC: (The international Union of pure and applied chemistry): solubility defines, solubility as an analytical composition of a saturated solution expressed as a proportoon of a designated solute in a designated solvent. Extra or Additional solute will not dissolve in a saturated solution. The solubility may be indicated by different units like, concentration, molar, molar fraction, molar ratio etc.
The solubility of substance is depends on the used solvents, temperature and pressure.
The thermodynamic solubility of a drug in a solvent is” under equilibrium condition (equal), at a given temperature and pressure, the maximum amount of the most stable crystalline form that stay in solution in a given volume of the solvent”.
The solubility of drugs is expressed in several ways as ppm(parts per million) according to BP( British pharmacopoeia) mg/mL and miles/liter (M/L.
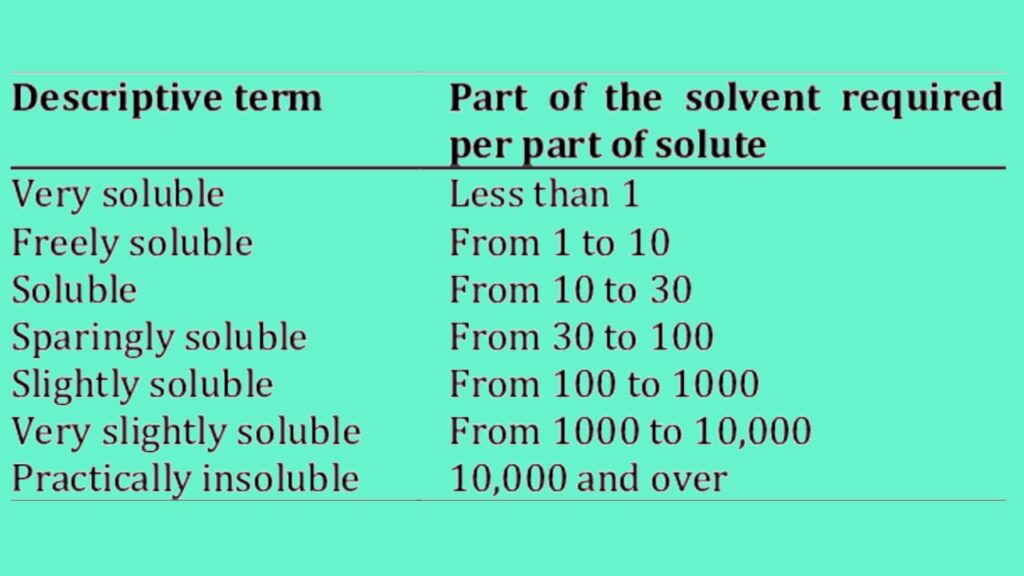
According to United state pharmacopoeia (USP) describe the solubility of drug is a part of the solvent required for part of the solute.
USP describe the solubility using as the several ways: