Bioavailability
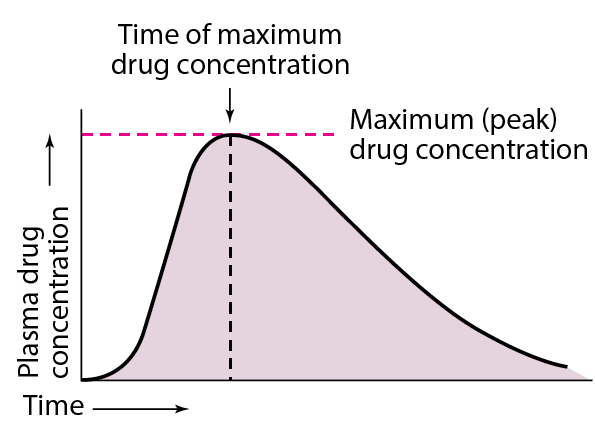
Bioavailability refers to the rate and extent of absorption of a drug from a dosage form as determined by its concentration-time curve in blood or by its excretion in urine. It is a measure of the fraction of administered dose that reaches the systemic circulation in the unchanged form.
Bioavailability of drug injected i.v. is 100%, but is frequently lower after oral ingestion because—
(a) the drug may be incompletely absorbed.
(b) the absorbed drug may undergo first-pass metabolism in the intestinal wall/liver or be excreted in bile.
Incomplete bioavailability after s.c. or i.m. the injection is less common but may occur due to local binding of the drug.
Bioequivalence
Oral formulations of a drug from different manufacturers or different batches from the same manufacturer may have the same amount of the drug (chemically equivalent) but may not yield the same blood levels—biologically inequivalent. Two preparations are considered bioequivalent when the rate and extent of bioavailability of the active drug from them are not significantly different.
Before a drug administered orally in a solid dosage form can be absorbed, it must break into individual particles of the active drug (disintegration). Tablets and capsules contain a number of other materials—diluents, stabilizing agents, binders, lubricants, etc. The released drug must then dissolve in the aqueous gastrointestinal contents. The rate of dissolution is governed by the inherent solubility, particle size, crystal form, and other physical properties of the drug. Differences in bioavailability may arise due to variations in disintegration and dissolution rates.
Bioavailability differences are especially noticeable in poorly soluble and slowly absorbed medicines. The rate of aspirin absorption is increased when the particle size is reduced (microfine tablets). If the drug particle is microfine, the amount of griseofulvin and spironolactone in the tablet can be cut in half. The particle size of readily water-soluble medicines, such as paracetamol, does not need to be reduced.
Bioavailability variation is important for medicines with a narrow safety margin (digoxin) or when accurate dose management is required (oral hypoglycaemics, oral anticoagulants). It might also be to blame for an antibiotic regimen’s success or failure. However, bioavailability variations are minor in many medicines, and the dangers of switching from a branded to a generic or another brand of the same drug are frequently overstated.
Make sure check our amazing article: Drug absorption in pharmacokinetics
References Book:
Essentials of Medical Pharmacology Seventh Edition by KD TRIPATHI MD