Penicillin is the most important class of antibiotic drugs of Beta lactam antibiotic drugs, this is the antibiotic drugs which are bactericidal for both gram positive and gram Negative.
It is the combination of 4 membered Beta- Lactam ring and 5 membered thiazolidine ring.
Table of Contents
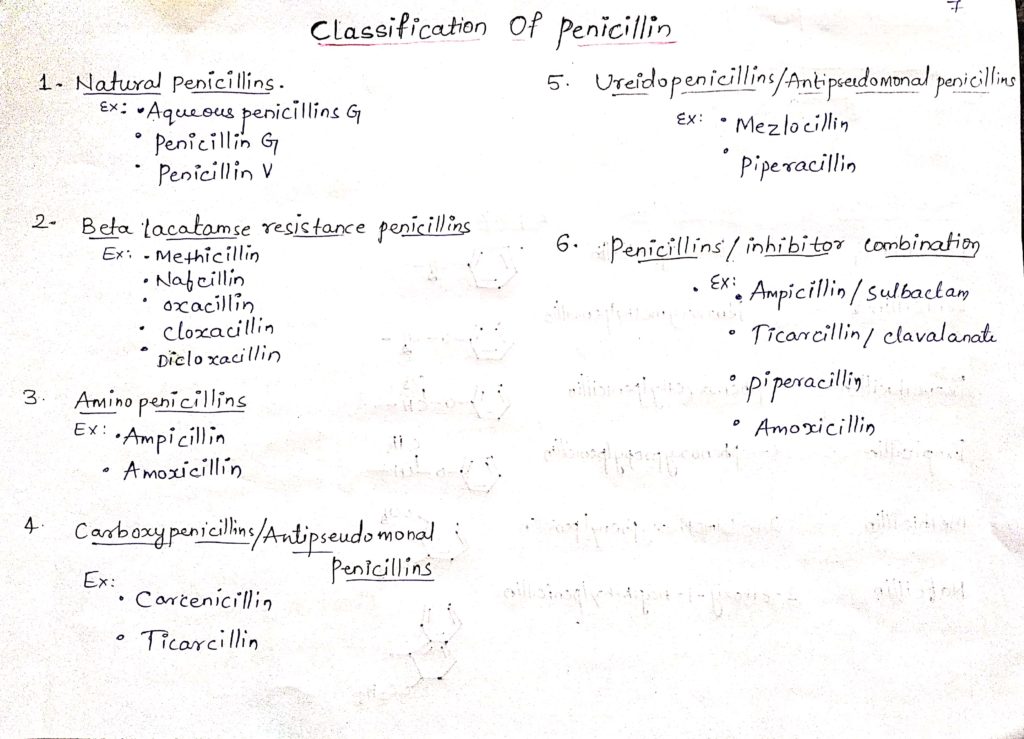
classification of penicillin:
Structure of penicillin:
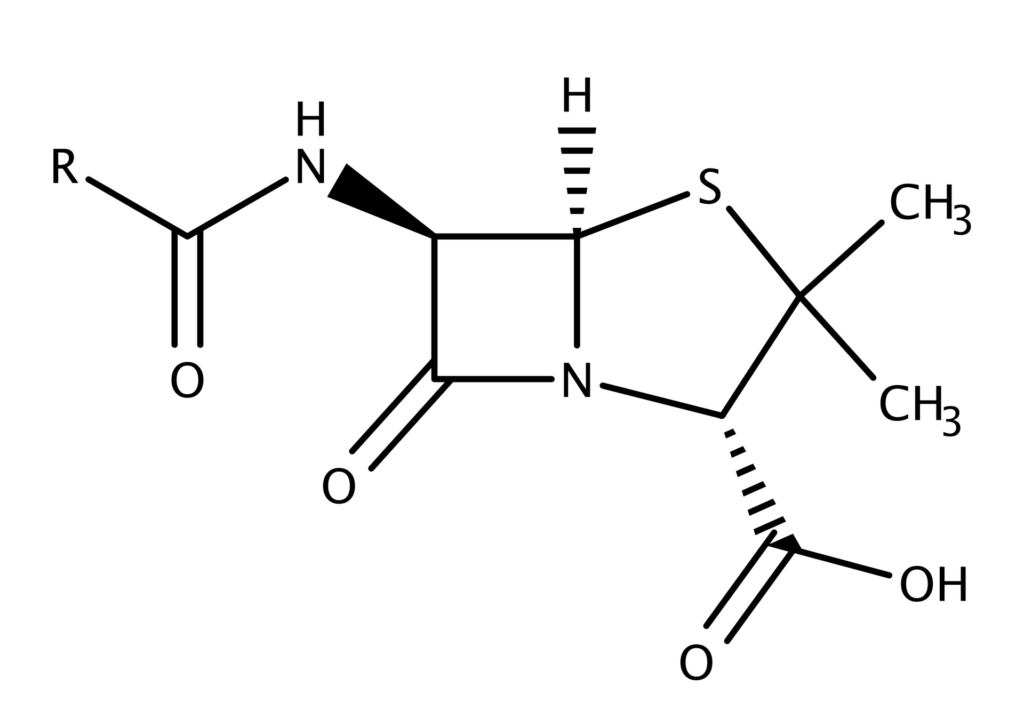
At 6 position of the general structure of penicillin attached of different R groups.
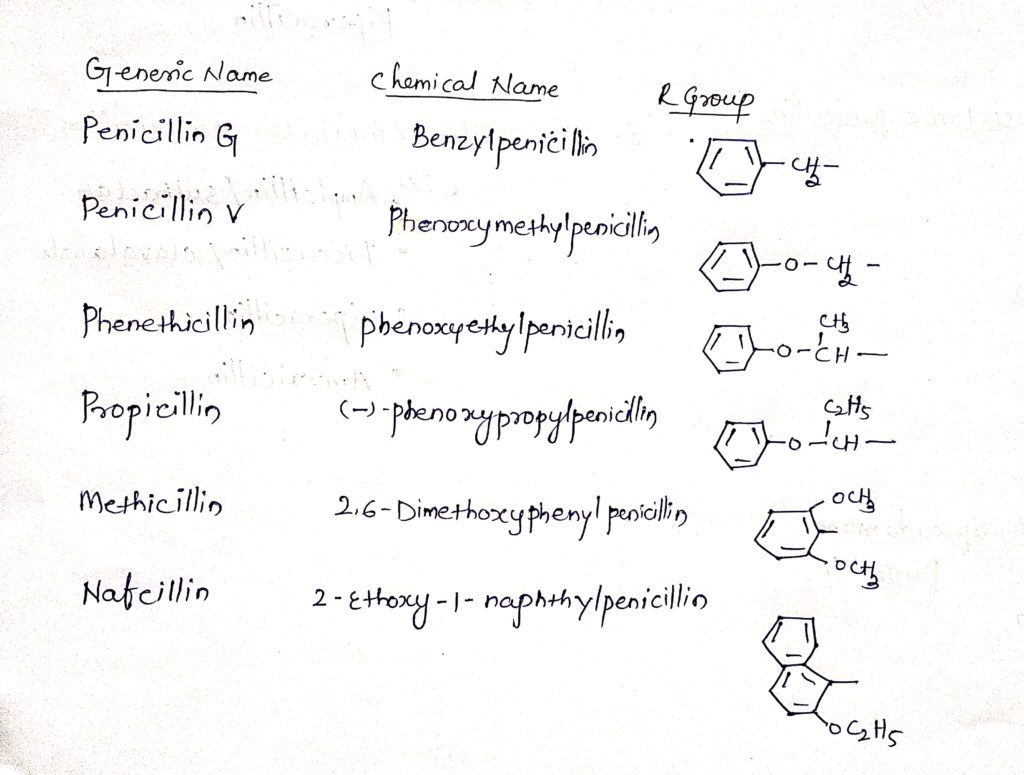
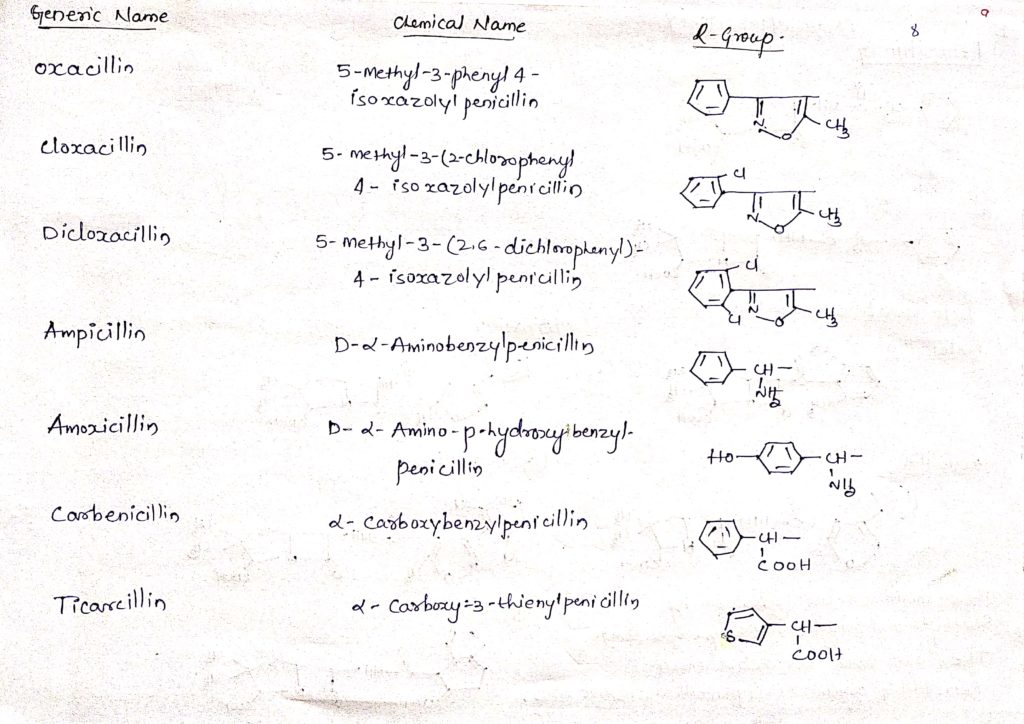
Important penicillin drugs along with their chemical structure:
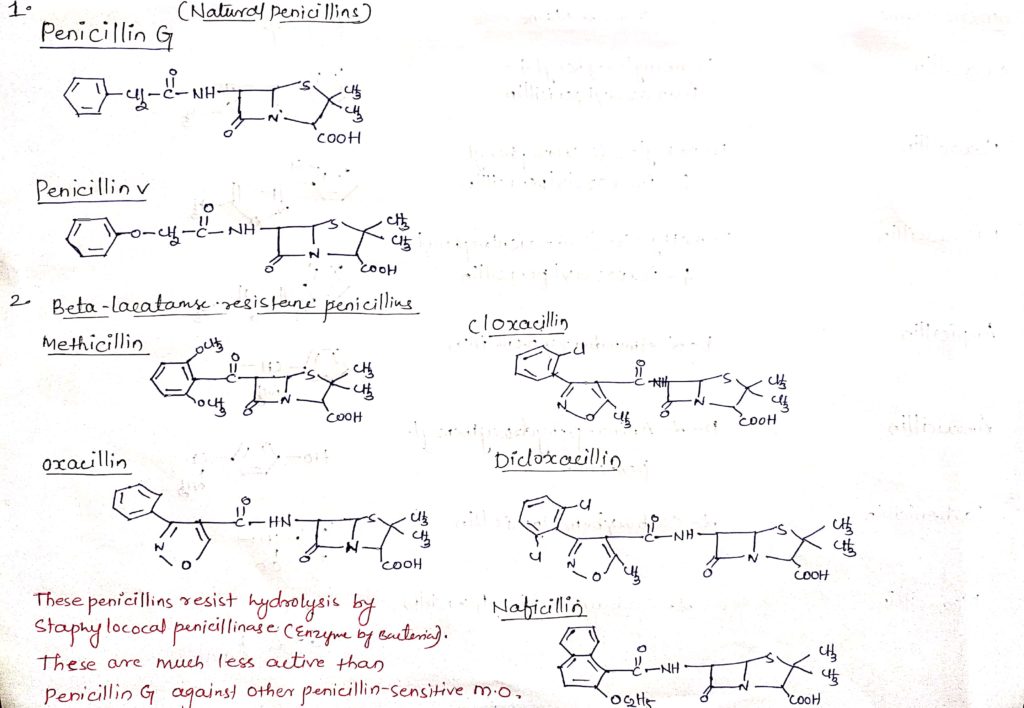
This penicillin resist hydrolysed by staphylococcus penicillin (enzyme by bacteria). These are much less active than penicillin G against other penicillin sensitive micro-organisms.
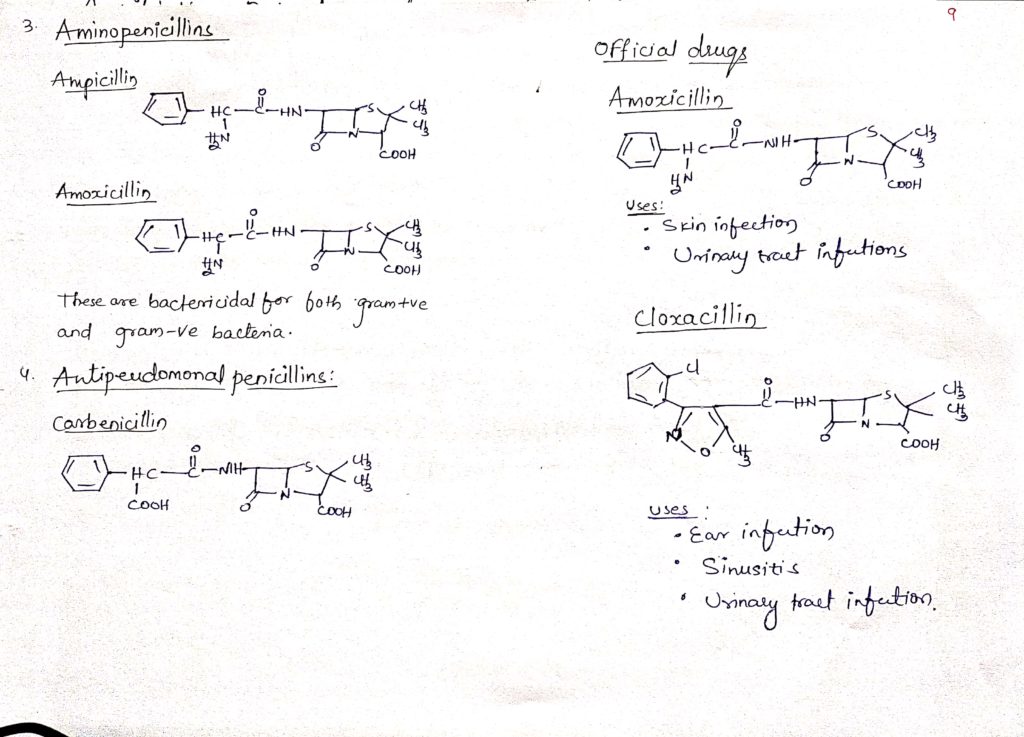
This types of penicillin are bactericidal for both gram positive and gram Negative bacteria.
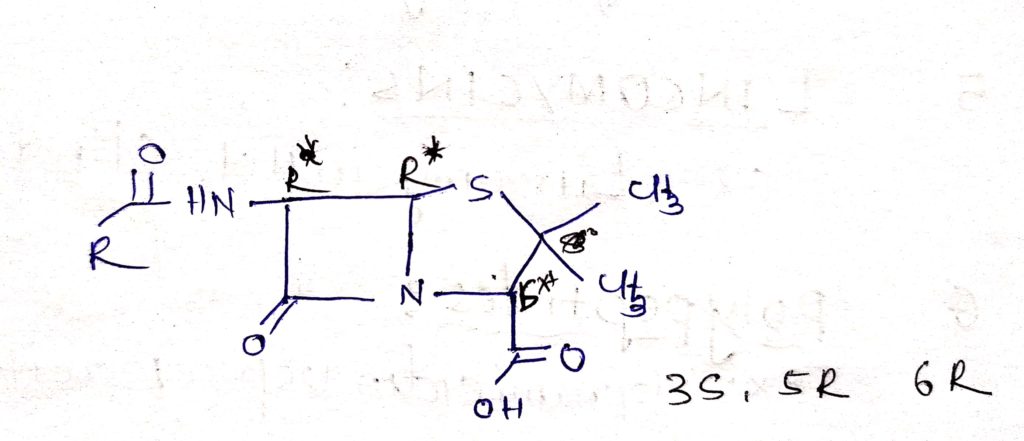
Stereochemistry of penicillin:
- penicillin is combined with highly unstable bicyclic system ( 4-membered Beta-Lactam) is fused with 5-membred thiazolidine.
- Penicillin molecule is contain 3 chiral carbon atoms( c-3 c-5 c-6) ( chiral: molecule or ion is chiral if it cannot be superposed on its mirror image by any combination of rotation and translation)
- Same absolute configuration about all the three centres it’s may naturally occuring and microbiologically active synthetic and semisynthetic penicillin.
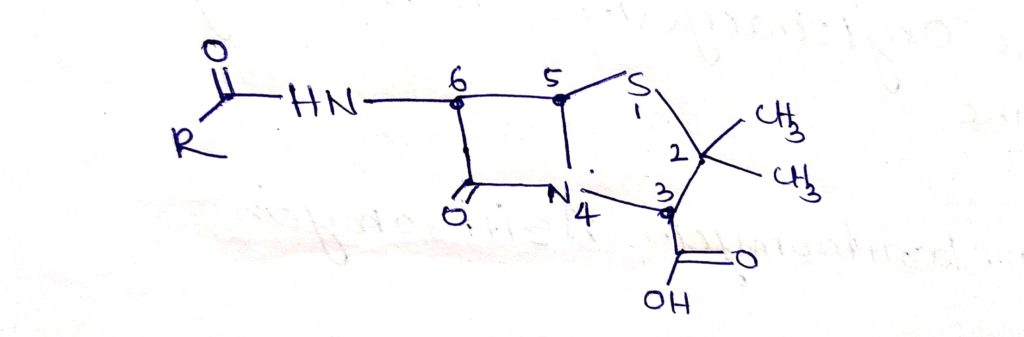
The carbon atom bearing the acylamino group (c-6) has the L configuration, where as the carbon to which the carboxyl groups is attach has the D- configuration.
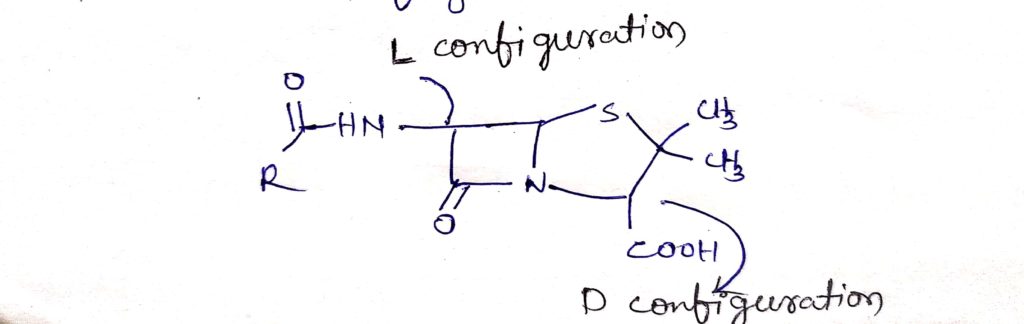
The acylamino and carboxyl group are trans to each other which is relative to penam ring system. The absolute stereochemistry of the penicillin is designated 3s:5R:6R