Acute Inflammation:
The changes in acute inflammation can be conveniently described under the following two headings.
- I) Vascular Events
- a. Haemodynamic changes.
- b. Altered vascular permeability.
- II) Cellular Events
- a. Exudation of leukocytes.
- b. Phagocytosis.
Table of Contents
I) Vascular Events:
Alteration in the microvasculature (arterioles, capillaries, and venules) is the earliest response to tissue injury. These alterations include hemodynamic changes and changes in vascular permeability.
a. Haemodynamic changes:
The earliest features of inflammatory response result from changes in the vascular flow and caliber of small blood vessels in the injured tissue.
The sequence of these changes is as under.
1. Irrespective of the injury, the immediate vascular response is the transient vasoconstriction of arterioles. With the mild form of injury, the blood flow may be re-established in 3-5 seconds. While with more severe injury, the vasoconstriction may last for about 5 min.
2. Next follows persistent progressive vasodilatation which involves mainly the arterioles and lesser extents to venules and capillaries. This change is obvious within half an hour of injury. Vasodilatation results in increased blood volume in the microvascular bed of the area, which is responsible for redness and warmth at the site of acute inflammation.
3. Progressive vasodilatation in turn may elevate the local hydrostatic pressure resulting in transudation of fluid into the extracellular space. This is responsible for swelling at the local site of acute inflammation.
4. Slowing or stasis is attributed to increased permeability of microvasculature that results in increased concentration of red cells and thus raised blood viscosity.
5. Slowing or stasis is followed by leukocyte’s margination or peripheral orientation of leukocytes (mainly neutrophils) along the vascular endothelium. The leucocytes stick to the vascular endothelium briefly, and then move and migrate through the gaps between the endothelial cells into the extravascular space known as emigration.
The Lewis experiment best demonstrates the characteristics of haemodynamic alterations in inflammation. Lewis used a firm stroking with a blunt tip to cause alterations in the skin of the inner forearm. A triple response, often known as a red line response, is a reply that has the following elements:
- A red line forms a few seconds after stroking and is caused by local capillary and venule dilatation.
- Flares are a brilliant reddish flush that surrounds the red line and is caused by vasodilation of the neighbouring arterioles.
- Wheal is an oedema or swelling of the surrounding skin caused by fluid transudation into the extravascular space.
b. Altered vascular permeability
In acute inflammation, the normally nonpermeable endothelial layer of microvasculature becomes leaky. This is explained by one or more of the following mechanisms.
Contraction of endothelial cells
This is the most common cause of increased venule leakiness, which only affects venules, leaving capillaries and arterioles unchanged. Due to their contraction, endothelial cells generate brief gaps between them, resulting in vascular leakiness. The release of histamine, bradykinin and other chemical mediators are involved. The reaction starts soon after the damage, is usually reversible, and lasts only a few minutes (15-30 minutes).
Retraction of endothelial cells
In this mechanism, there is the structural reorganization of the cytoskeleton of endothelia cells that causes reversible retraction at the intercellular junctions. This change affects the venules and is mediated by cytokines such as Interleukin-1 (IL-1) and tumor necrosis factor α (TNF-α). The onset of response takes 4-6 hours after injury and lasts for 2-4 hours or more.
Direct injury to endothelial cells
Cell necrosis and the formation of physical holes at the sites of detached endothelial cells result from direct insult to the endothelium. The thrombosis process begins at the location of injured endothelial cells. The alteration has an impact on the microvasculature at all levels. The increased permeability may appear immediately after damage and remain for several hours or days (immediate sustained leakage), or it may appear after a 2-hour delay and last for hours to days (delayed sustained leakage) (delayed prolonged leakage).
Endothelial injury mediated by leukocytes
Leukocyte adhesion to the endothelium at the site of inflammation may result in leukocyte activation. Proteolytic enzymes and hazardous oxygen species are released by active leukocytes, which may cause endothelial damage and increased vascular leakiness. This type of vascular leakiness affects only the venules and is a delayed response.
Other Mechanisms
In addition, the newly formed capillaries during the process of repair are excessively leaky.
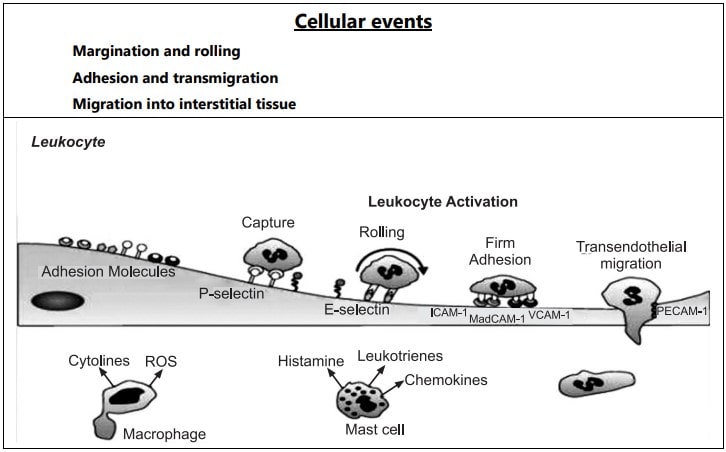
II) Cellular Events
a. Exudation of leukocytes:
The most essential component of the inflammatory response is the escape of leukocytes from the lumen of the microvasculature to the interstitial tissue. Polymorphonuclear neutrophils are the initial line of defense in acute inflammation, followed by monocytes and macrophages. The following are the alterations that cause leukocyte migration:
Changes in the formed elements of blood:
In the early stage of inflammation, the rate of flow of blood increases due to vasodilation. But subsequently, there is slowing or stasis of the bloodstream. With stasis, changes in the normal axial flow of blood in the microcirculation take place. The normal axial flow consists of a central stream of cells comprised by leukocytes and RBC and a peripheral cell-free layer of plasma close to the vessel wall. Due to slowing and stasis, the central stream of cells widens and the peripheral plasma zone becomes narrower because of loss of plasma by exudation. This phenomenon is known as ‘margination’. As a result of redistribution, the neutrophils of the central column come close to the vessel wall, this is known as ‘pavementing’.
Rolling and Adhesion
Peripherally marginated and pavemented neutrophils slowly roll over the endothelial cell lining of the vessel wall (Rolling phase). Neutrophils first roll among the surface of the endothelium in a process mediated by selectins, adhesion molecules that are expressed by endothelial cells and that bind reversibly to sites on the leukocyte membrane. Later, neutrophils become firmly adherent to the endothelium by binding of leukocyte adhesion molecules (Selectins, Integrins, Intercellular adhesion molecules-1, vascular cell adhesion molecule-1, platelet endothelial cell adhesion molecule-1, etc.) This is followed by the transient bond between leukocytes and endothelial cells becoming firmer. (Adhesion phase). The following adhesion molecules bring about rolling and adhesion phases.
Transmigration
After sticking neutrophils to the endothelium, the former move along the endothelial cell is found where the neutrophils throw out cytoplasmic pseudopods. Subsequently, the neutrophils are lodged between the endothelial cells and cross the basement membrane by damaging it locally with secreted collagenase and escape out into the extravascular space. This is known as ‘transmigration’. The damaged basement membrane is repaired almost immediately, simultaneous to the emigration of leukocytes, escape of RBC’s through the gaps between the endothelial cells. It is a passive phenomenon, RBCs being forced out either by raised hydrostatic pressure or may escape through the endothelial defects left after the emigration of leukocytes.
Chemotaxis
The movement of leukocytes from the vessel lumen into a damaged area is called chemotaxis and is mediated by substances known as chemotactic factors that diffuse from the area of tissue damage. All granulocytes and monocytes respond to chemotactic factors and move along a concentration gradient. Chemo attractants can be exogenous or endogenous. Most exogenous chemotactic factors are bacterial or other microbial products. There are many endogenous chemotactic factors like fibrin, fibrinopeptides, bacterial components, IL-8, IL-5, histamine, monocyte chemotactic factors (MCP-1 & MCP-5).
b. Phagocytosis
It is defined as “the process of engulfment of solid particulate material by the cells”. There are two main types of phagocytic cells:
• Polymorphonuclear neutrophils (PMN’s) which appear early in acute inflammatory response also called as microphages.
• Circulating Monocytes and fixed tissue mononuclear phagocytes are called macrophages.
Neutrophils and macrophages on reaching the tissue space produce several proteolytic enzymes lysozyme, protease, collagenase, elastase, lipase, proteinase, gelatinase, and acid hydrolases. These enzymes degrade collagen and the extracellular matrix that will kill bacteria.
Also read: Etiology of Inflammation