The atom is the smallest component of an element that is capable of maintaining stability. An element is a chemical compound that only includes atoms of the same kind; for example, iron only has iron atoms. Multiple atom types can coexist in a molecule. For example, water is a complex that contains both hydrogen and oxygen atoms.
An atom is electrically neutral if the amount of positively charged protons in the nucleus equals the number of negatively charged electrons orbiting the nucleus.
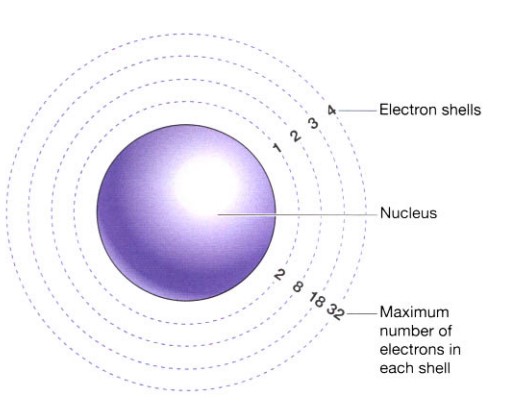
Table of Contents
Atomic number and atomic weight
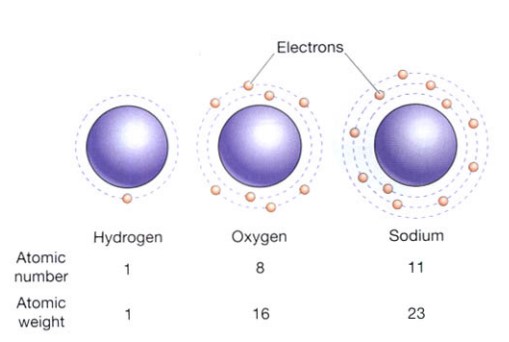
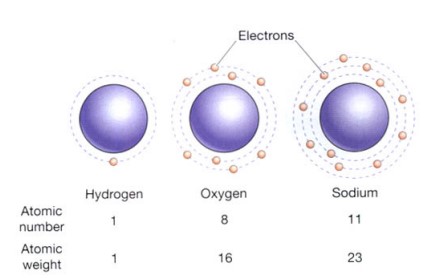
The number of protons in an element’s atomic nucleus determines how different it is from other elements. For instance, sodium has 11 protons per nucleus, compared to 8 for oxygen and 1 for hydrogen. The atomic number of hydrogen, oxygen, and sodium are consequently 1, 8, and 11 correspondingly. The atomic number is the quantity of protons in the nucleus of an atom. Consequently, each element has a unique atomic number (Figure is shown below). The protons and neutrons in the atomic nucleus of an element are added to determine its atomic weight (Figure is shown below).
The electrons are depicted as being in concentric rings around the nucleus in the illustration below. The electrons’ various energies in regard to the nucleus are diagrammatically represented by these shells, not by their actual positions. Only two electrons can fit into the first energy level, so it fills up first. Next, the second energy level, which can only store eight electrons, is filled. The third energy level and higher energy levels each contain more electrons than the levels before them.
The electron configuration denotes the distribution of the electrons in each element, e.g. sodium is 2 8 1
When an atom’s outermost electron shell is completely filled, it is most stable. Once the first two shells are full of electrons, the atom can achieve stability by having either all 18 electrons or precisely eight, in the third shell. The enormous variety of complex molecules that make up life is made up of reactive atoms that mix with other reactive atoms when the outermost shell does not contain a steady quantity of electrons. The section on molecules and compounds will go into greater detail about this.
Isotopes
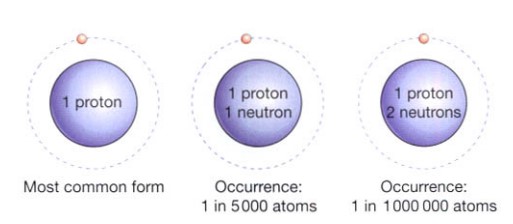
These are atoms of an element whose nucleus contains a distinct number of neutrons. Since neutrons have no electrical charge, this has no effect on the electrical activity of these atoms, but it does change their atomic weight. The hydrogen atom, for instance, comes in three different states. The most prevalent form has one circling electron and one proton in the nucleus. One proton and one neutron make up the nucleus of a different form. One proton, two neutrons, and an orbiting electron make up the nucleus of the third form. Isotopes are the name for these three varieties of hydrogen.
Taking into account the isotopes of hydrogen and the proportions in which they occur, the atomic weight of hydrogen is 1.008, although for many practical purposes it can be taken as 1.
Molecules and compounds
It was mentioned earlier that the atoms of each element have a specific number of electrons around the nucleus. When the number of electrons in the outer shell of an element is the optimum number the element is described as inert or chemically unreactive, i.e. it will not easily combine with other elements to form compounds. These elements are inert or noble gases —helium, neon, argon, krypton, xenon, and radon.
Molecules consist of two or more atoms that are chemically combined. The atoms may be of the same element, e.g. a molecule of atmospheric oxygen (O2) consists of two oxygen atoms. Most molecules, however, contain two or more different elements; e.g. a water molecule (H, O) contains two hydrogen atoms and an oxygen atom. As mentioned earlier, when two or more elements combine, the resulting molecule can also be referred to as a compound. Compounds that contain the element carbon are classified as organic, and all others as inorganic. The body contains both.
Covalent and ionic bonds
The myriad of chemical reactions that make up how the body works are entirely dependent on how atoms combine, bind, and dissociate. For instance, the fundamental building block of all life on Earth is the uncomplicated water molecule. Human biology could never have developed if water was a less stable substance and the atoms were more easily dissociable. In contrast, the body relies on the breakdown of different substances (such as carbohydrates and fats) to provide energy for cellular functions. Atoms establish chemical bonds when they come together, and these bonds can either be covalent or ionic in nature.
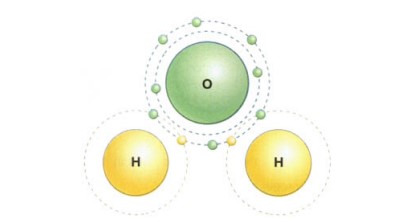
Atoms share their electrons with one another to create covalent bonds. Because atoms are most stable when their outer electron shells are filled, the majority of atoms employ this kind of bond when they come together; it creates a powerful and stable connection between them. Covalent bonding is used to construct the water molecule. Although hydrogen only contains one electron in its outer shell, two electrons are the ideal number for this shell. Although oxygen’s outer shell includes six electrons, eight electrons are the ideal number for this shell. Therefore, each hydrogen atom will share its electron with the oxygen atom when one oxygen atom and two hydrogen atoms come together. This gives the oxygen atom a total of eight outside electrons and confers stability. Each of the two hydrogen atoms receives one of the oxygen atom’s electrons, giving each hydrogen atom two electrons in its outer shell, making them both stable.
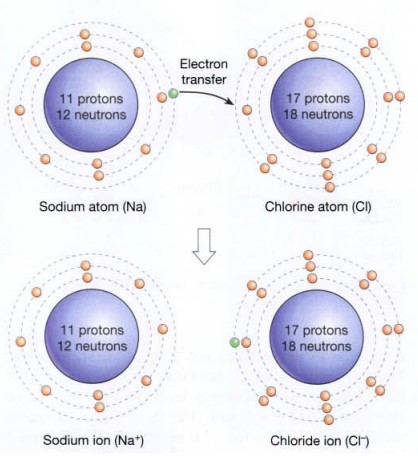
When electrons are moved from one atom to another, ionic bonds, which are weaker than covalent connections, are created. For instance, the only electron in the sodium atom’s outer shell is transferred to the outer shell of the chlorine atom when sodium (Na) and chlorine (Cl) combine to produce sodium chloride (NaCl).
Electrolytes
An ionic compound, e.g. sodium chloride, in solution in water is called an electrolyte because it can conduct electricity. Electrolytes are important body constituents because:
- Some conduct electricity, essential for muscle and nerve function
- Some exert osmotic pressure, keeping body fluids in their own compartments
- Some function in acid-base balance, as buffers to resist pH changes in body fluids.
In this discussion, sodium chloride has been used as an example of the formation of an ionic compound and to illustrate electrolyte activity. There are, however, many other electrolytes within the human body which, though in relatively small quantities, are equally important. Although these substances may enter the body in the form of compounds, such as sodium bicarbonate, they are usually discussed in the ionic form, that is, as sodium ions (Na+ ) and bicarbonate ions (HCO3 – ).
Molecular weight
The molecular weight of a molecule is the sum of the atomic weights of the elements which form its molecules,
Water (H.OH)
2 hydrogen atoms
1 oxygen atom
(atomic weight 1) 2
(atomic weight 16) 16
Molecular weight = 18
Molar concentration
This is the term recommended in the Systeme Internationale for expressing the concentration of substances present in body fluids (SI units).
The mole (mol) is the molecular weight in grams of a substance (formerly called 1 gram molecule). One mole of any substance contains 6.023 x 1023 molecules or atoms. For example, 1 mole of sodium bicarbonate (the example above) is 84 grams.
Also, read; Internal communication