Table of Contents
Introduction to antigen-presenting cells
- Antigen-Presenting Cells (APCs) are a heterogeneous group of immune cells that mediate the cellular immune response by processing and presenting antigens for recognition by certain lymphocytes such as T cells.
- Large-molecular-weight proteins must be reduced to a length of 8 to 30 amino acids before being loaded with antigens into class I and II molecules.
- Antigens breathed, eaten, or injected into the body are internalised by specialised antigenpresenting cells (APCs) and processed in an endocytic pathway to produce tiny peptides.
- APCs can be divided into “professional” and “amateur” subsets.
- Professional APCs such as monocytes, macrophages, dendritic cells, and B cells are fully committed to antigen presentation as an integral part of their function in the generation of the immune response.
- Other cells, such as endothelial cells, fibroblasts, glial cells, pancreatic -cells, keratinocytes, and thyroid cells, present antigens only under select conditions.
Professional antigen-presenting cells
Monocytes and Macrophages
- Foreign materials are digested by monocytes and macrophages, and antigenic epitopes are presented to immunocompetent cells by these cells.
- Monocytes circulate in the bloodstream, whereas macrophages can be found in almost all organs, including the brain (microglia), bone (osteoclasts), and connective tissue (fibroblasts) (histiocytes).
- The biggest concentration of macrophages in the body is found in the kupffer cells of the liver.
- Both innate and adaptive immune responses are aided by macrophages.
- Antigens coupled to PAMP, Toll-like, scavenger, and mannose receptors activate macrophages as part of innate immunity.
- Antigens are identified in an adaptive response by receptors for intermediate molecules like antibodies and complement fragments.
Dendritic Cells
Steinman described a rare peripheral blood cell with membrane dendrites that resembled those of a neuron in 1973. Other research has shown that the dendritic cell (DC) is an antigen-producing cell that may be found in both lymphoid and nonlymphoid organs. Dendritic cell precursors originate from committed CD34+ stem cells in the bone marrow, develop into immature myeloid, monocytic, and lymphoid DCs, and then seed the blood. DC populations are challenging to detect due to variability in cell surface markers. However, a possible hematopoietic stem cell The DC differentiation route is depicted in the diagram below.
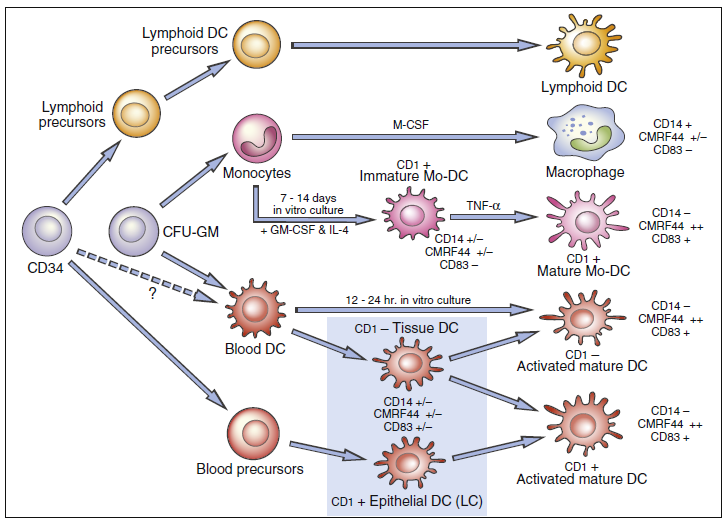
- Several DC subsets localize in tissues or circulate in peripheral blood.
- Two populations of dendritic cells are derived from myeloid stem cells: (1) One population migrates to the lymph node where it becomes follicular dendritic cells.
- Lymphoid or plasmacytoid dendritic cells localize in the T cell compartment within the lymph node.
- Monocyte precursors may remain in the peripheral blood as monocytic dendritic cells, although this concept is still being debated.
- Dendritic cells are uniquely suited for antigen presentation.
- High numbers are concentrated at likely sites of microbial entry into the body (e.g., the intestine and respiratory tract).
- Most DCs can efficiently capture and process antigens for presentation to T cells.
- Moreover, they are mobile and can migrate through tissue or stimulate immune responses in the regional lymph nodes.
Follicular Dendritic Cells
Follicular dendritic cells (FDCs) are located in the germinal follicles of lymph nodes and have a variety of activities, including B cell activation and immunologic memory preservation (given figure 5-2 below).
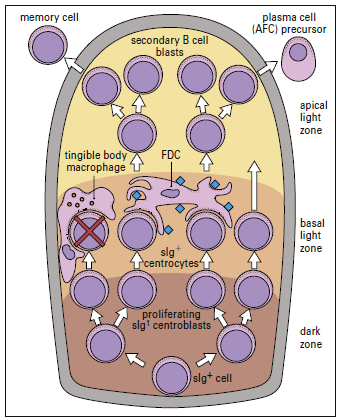
The functions of the germinal center are clonal proliferation
FDCs do not digest antigen for presentation to T lymphocytes, unlike other DCs. Rather, they use a novel method to trigger a CD4Th2 response and retain memory. FDCs bind processed and unprocessed antigens to beaded, three-dimensional structures called iccosomes via a variety of receptors (Figure 5-3).
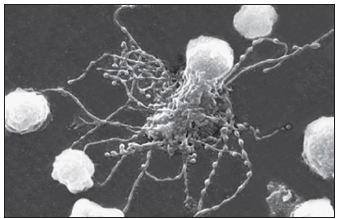
Antigens may be retained in iccosomes for months and Perhaps years. To stimulate antibody production, an antigen is slowly released, processed by B cells, and presented to T cells. in the context of class II molecules. Cytokines are produced from FDCs also contribute to the activation and differentiation of B cells into plasma cells. As a consequence of the continual stimulation of B cells, antibodies and memory cells are continually produced.
Interstitial Dendritic Cells
Interstitial dendritic cells are found in most tissue, except the brain and the eye. These cells serve as a sentinel for detecting foreign or antigenic molecules. The largest reservoir of IDCs resides in the skin, where resident Langerhans LCs occupy 25% of the skin surface area, but only 2% to 3% of skin cells. Resting or immature LCs reside in the suprabasilar epidermis and are identified by the presence of cytoplasmic Birbeck granules and by the expression of CD1, class I, class II molecules, and abundant receptors for pathogen-associated microbial patterns (PAMPs) and mannose. When antigens are captured, the immature LCs disengage from the epithelium and migrate to the paracortex or T cell zones of the regional lymph nodes. During migration, LCs transform into “veiled cells.” After entering the lymph node, the “veiled cells” assume the role of resident interdigitating dendritic cells that present antigen to naïve CD4 cells (Figure 5-4).
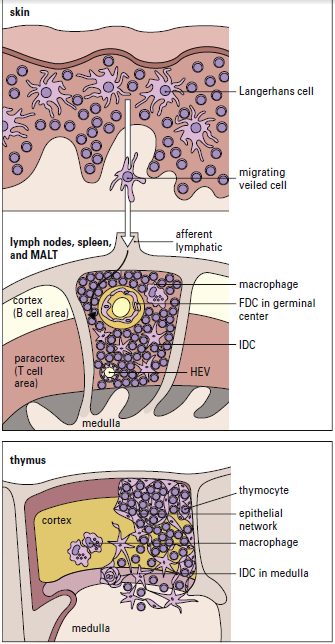
Lymph nodes in residence IDCs are also capable of processing soluble antigens that reach the lymph node through the lymph fluid. Soluble antigens in the lymph node percolate through the node in a way that assures interaction with IDCs. The antigen is delivered to naive and effector CD4 T cells, which are close to IDCs, after internalization and processing (Figure 5-5).
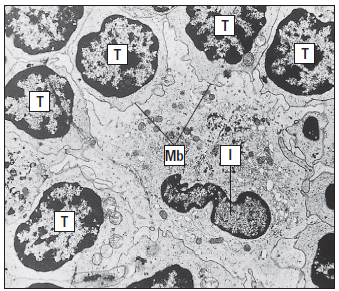
cells.
Plasmacytoid Dendritic Cells
Plasmacytoid dendritic cells (pDCs) resemble antibody-secreting plasma cells and are believed to arise from a lymphoid progenitor. Activated pDCs link innate immunity and adaptive immunity to viruses. In the innate response, Toll-like receptors 7 and 9 binds viral deoxyribonucleic acid (DNA) from herpes simplex virus (HSV), Sendai virus, human immunodeficiency virus type 1 (HIV-1), and influenza virus. Receptor activation of pDC’s initiates the synthesis of α-interferon and β-Interferon. Interferon prevents the spread of the virus to uninfected cells and also activates natural killer cells.
B Cells
B cells present antigens at 100 to 10,000 times lower concentrations than that required for macrophage presentation. Antigens bind to an antigen-specific B cell receptor (BCR), which is a modified antibody. Cross-linking of BCRs internalizes antigen, which is degraded in the cytoplasm and presented to T cells in context with class II markers.