Table of Contents
Presentation of exogenous antigens
Exogenous antigens: macrophages and dendritic cells bind antigens using a number of different receptors for PAMPs, complement fragments, or antibodies. Opsonized bacteria and antigens are ingested by a process called phagocytosis. The mechanism for class II molecule delivery differs with macrophages and B cells.
B cells use receptor-mediated endocytosis to ingest foreign material. B cell receptors are localized in areas containing membrane clathrin. Receptor binding activates the clathrin and facilitates an inward folding of the cell membrane to form a vesicle. After recycling the clathrin molecules and the B cell receptors to the cell surface, the vesicle becomes a membrane-bound vacuole.
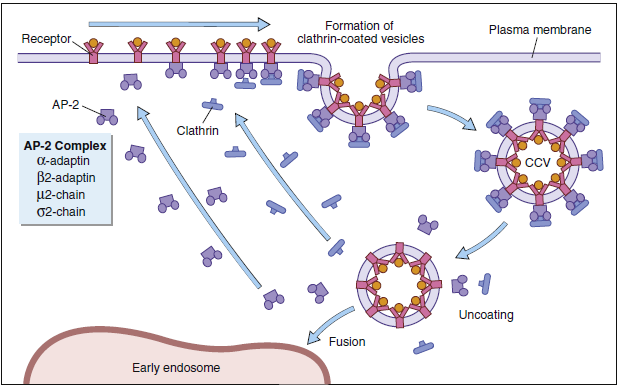
Different terms are used to describe the vacuoles in macrophages and B cells. In monocytes and macrophages, the vacuole is called a phagosome. Cytoplasmic lysosomal vesicles containing hydrolytic enzymes fuse with the phagosome membrane and empty their contents into the phagosome, which is now termed a phagolysosome. In B cells, endocytosis forms a vacuole called an early endosome. In the endocytic pathway, early endosomes travel through a series of tubes and vesicles from the periphery to deep inside the cell (late endosome).
Class II molecules synthesized in the endoplasmic reticulum rapidly associate with a 30-kilodalton (kDal) invariant peptide chain (Ii). Homotrimers of the Ii chain associate with three α-heterodimers or β-heterodimers of class II molecules. The Ii has two roles in antigen presentation: (1) It contains a signaling sequence that directs the class II molecule—into the endosome of the phagolysosome. (2) The Ii also prevents the loading of peptides into the binding groove until class II molecule enters the endosome or the phagolysosome. The Ii may also contribute to the formation of the binding cleft and the overall structure of the class II molecule recognized by cells. After entry into an antigen-containing endosome or phagolysosome, the Ii is removed in an orderly proteolytic reaction.
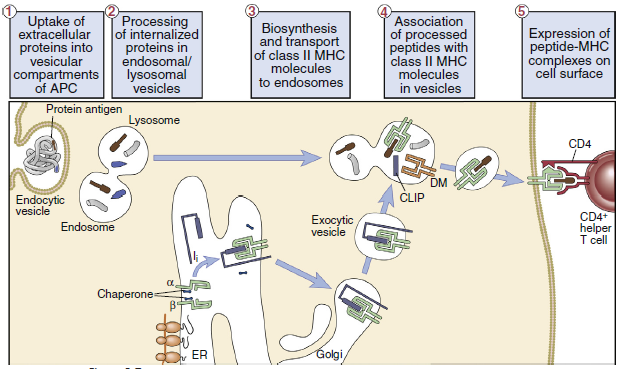
In the endosome or phagolysosome, the invariant chain is truncated to a 3-kDal peptide called the class II-associated invariant chain peptide, or CLIP. In humans, the disassociation of the CLIP is facilitated by the HLA-DM (monocytes) or the HLA-DO (B cells), which also stabilize the class II molecule and assist in peptide selection.
Stabilization allows the binding of peptides with low and high structural stability. Low-stability peptides are released from class II molecules. The interaction between high-stability peptides and the binding groove on class II molecules create a stable peptide–class II molecule complex. Large and small peptides may bind to the open-ended class II binding cleft. Proteolytic enzymes trim the larger peptide to 10 to 30 amino acids.
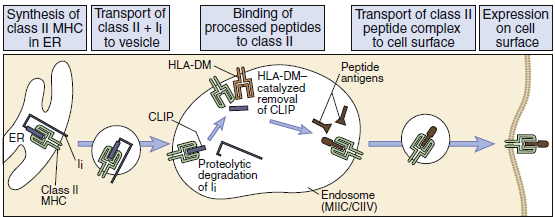
leukocyte antigen (HLA)–DM.
Dendritic cells and cancer vaccines
Because HLA molecules are downregulated in tumour cells, tumour-specific antigens cannot be delivered to T cells, tumour cells are typically not immunogenic. Dendritic cell cross-priming is a technique used by vaccine researchers to boost the immunogenicity of tumour cells for use in vaccines. Dendritic and tumour cells are isolated from the patient during cross-priming. DCs and tumour cells are combined together in the lab and cultured for many days. Intact tumour cells are ingested by DCs, which then digest the antigens for presentation in the context of class I molecules. The patient’s CD8 cytotoxic cell response to tumour cells is elicited by the administration of antigen-pulsed dendritic cells.
This concept is being used to develop autologous pulsed dendritic cell vaccines against tumors. From a theoretical perspective, dendritic cell vaccines could be used to treat all cancers. However, clinical experience shows that at the present time, the efficacy of dendritic cell vaccination is limited to melanoma and renal cancer treatments.
Dendritic cells and disease
Dendritic cells may play a critical role in psoriasis. The interaction between dendritic cells and T cells plays a critical role in the formation of plaques in chronic psoriasis. Plaques contain high numbers of pDCs, myeloid DCs, and inflammatory dendritic skin cells. Interactions between DCs and CD4 cells This led to the clonal expansion of CD8 cells in skin lesions. CD8 These cells produce proinflammatory cytokines that are implicated in in psoriasis. Dendritic cells also play a role in the pathogenesis of infections. human immunodeficiency virus (HIV) infections. FDCs can concentrate infectious viruses for extended periods. Moreover, FDCs promote the migration of T cells into the germinal center and the transfer of viruses to T cells. At the same time, At that time, IDCs are infected and support viral replication for 45 to 60 days.
Make sure also check out our amazing article: Antigen-Presenting Cells