Acids, alkalis, and pH: the number of hydrogen ions present in a solution is a measure of the acidity of the solution. The maintenance of the normal hydrogen ion concentration ([ H+]) within the body is an important factor in maintaining a stable environment, i.e. homeostasis.
Table of Contents
The pH scale-Acids, alkalis, and pH
A standard scale for the measurement of the hydrogen ion concentration in solution has been developed: the pH scale. Not all acids ionize completely when dissolved in water. The hydrogen ion concentration is a measure, therefore, of the amount of dissociated acid (ionized acid) rather than of the total amount of acid present. Strong acids dissociate more freely than weak acids, e.g. hydrochloric acid dissociates freely into H+ and Cl~, while carbonic acid dissociates much less freely into H+ and HCO3 -. The number of free hydrogen ions in a solution is a measure of its acidity rather than an indication of the type of molecule from which the hydrogen ions originated.
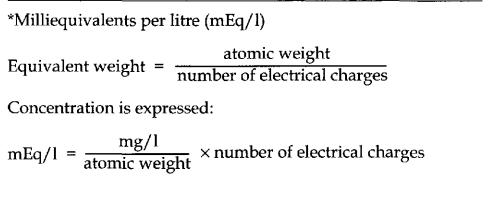
The alkalinity of a solution depends on the number of hydroxyl ions (OH-). Water is a neutral solution because every molecule contains one hydrogen ion and one hydroxyl radical. For every molecule of water (H.OH) which dissociates, one hydrogen ion (H+ ) and one hydroxyl ion (OH-) are formed, neutralizing each other.
The scale for the measurement of pH was developed taking water as the standard.
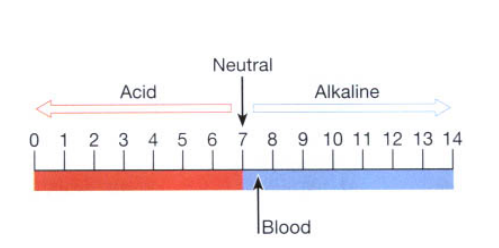
In a neutral solution such as water, where the number of hydrogen ions is balanced by the same number of hydroxyl ions, the pH = 7. The range of this scale is from 0 to 14.
A pH reading below 7 indicates an acid solution, while readings above 7 indicate alkalinity (Fig. 2.6). A change of one whole number on the pH scale indicates a tenfold change in [H+ ]. Therefore, a solution of pH 5 contains ten times as many hydrogen ions as a solution of pH 6.
Ordinary litmus paper indicates whether a solution is an acid or alkaline by coloring blue for alkaline and red for acid. Other specially treated absorbent papers give an approximate measure of pH by a color change. When accurate measurements of pH are required, sensitive pH meters are used.
pH values of the body fluids
Body fluids have pH values that must be maintained within relatively narrow limits for normal cell activity. The pH values are not the same in all parts of the body; e.g. the normal range of pH values of certain body fluids are shown in the table below.
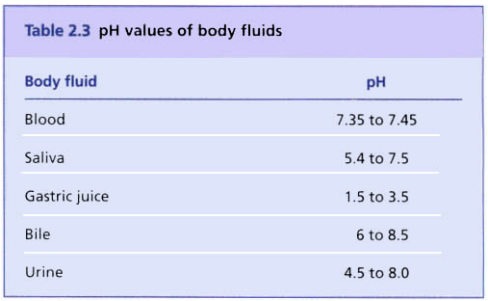
The pH value of an organ is produced by its secretion of acids or alkalis, which establishes the optimum level. The high acid pH of gastric juice is maintained by hydrochloric acid secreted by the parietal cells in the walls of the gastric glands. The low pH value in the stomach provides the environment best suited to the functioning of the enzyme pepsin, which begins the digestion of dietary protein. Saliva has a pH of between 5.4 and 7.5, which is the optimum value for the action of salivary amylase, the enzyme present in saliva that initiates the digestion of carbohydrates. The action of salivary amylase is inhibited when food containing it reaches the stomach and is mixed with acidic gastric juice.
Blood has a pH value between 7.35 and 7.45. The pH range of blood compatible with life is 7.0 to 7.8. The metabolic activity of the body cells produces certain acids and alkalis which alter the pH of the tissue fluid and blood. To maintain the pH within the normal range, there are substances present in blood that act as buffers.
Buffers-Acids, alkalis, and pH
The optimum pH level is maintained by the balance between acids and bases produced by cells. Bases are substances that accept (or bind) hydrogen ions and when dissolved in water they produce an alkaline solution. Buffers are substances such as phosphates, bicarbonates, and some proteins that maintain the [H+ ] within normal, but narrow, limits. Some buffers ‘bind’ hydrogen ions and others ‘bind’ hydroxyl ions, reducing their circulating levels and preventing damaging changes. For example, if there is sodium hydroxide (NaOH) and carbonic acid (H2CO3) present, both will ionize to some extent, but they will also react together to form sodium bicarbonate (NaHCO3) and water (H.OH). One of the hydrogen ions from the acid has been ‘bound’ in the formation of the bicarbonate radical and the other by combining with the hydroxyl radical to form water.
NaOH (sodium hydroxide) + H2 CO3 (Carbonic acid) → NaHCO3 (sodium bicarbonate) + H.OH (water)
Acidosis and alkalosis-Acids, alkalis and pH
The substances in the complex buffer system that ‘bind’ hydrogen ions are called the alkali reserves of the blood. When the pH is below 7.35, and all the reserves of the alkaline buffer are used up, the condition of acidosis exists. When the reverse situation pertains, the pH is above 7.45, and the increased alkali uses up all the acid reserves, the state of alkalosis exists.
The buffer systems maintain homeostasis by preventing dramatic changes in the pH values in the blood, but can only function effectively if there are some means by which excess acid or alkali can be excreted from the body. The organs most active in this way are the lungs and the kidneys. The lungs are important regulators of blood pH because they excrete carbon dioxide (CO2). CO2 increases [H+] in body fluids because it combines with water to form carbonic acid, which then dissociates into a bicarbonate ion and a hydrogen ion.
CO2 (Carbon dioxide) +H 2O (water) →H2 CO3 (carbonic acid) →H+ (Hydrogen ion) + HCO3– (Bicarbonate ion)
In acidosis, the brain detects the rising [H+] in the blood and stimulates breathing, causing increased CO2 loss and a fall in [H+]. Conversely, in alkalosis, the brain can reduce the respiration rate to increase CO2 levels and increase [H+], restoring pH to normal.
The kidneys can form ammonia, an alkali, which combines with the acid products of protein metabolism and is then excreted in the urine. The buffer and excretory systems of the body together maintain the acid-base balance so that the pH range of the blood remains within normal but narrow limits.
Also read: Atoms, molecules, and compounds