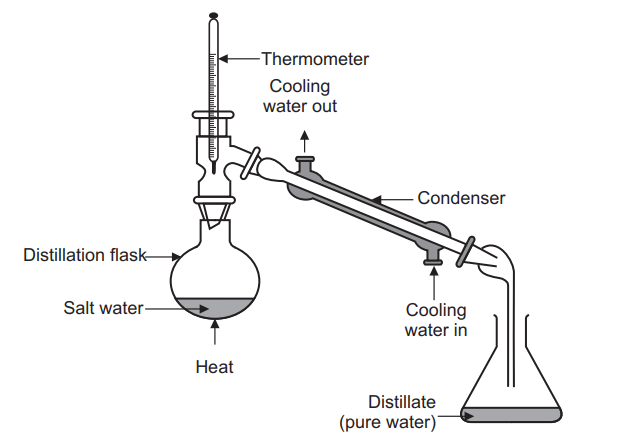
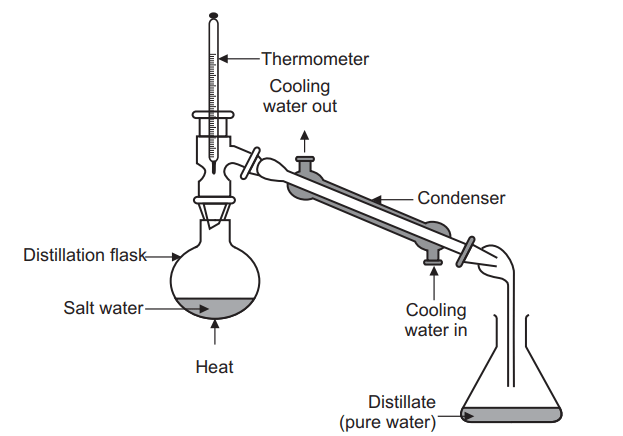
Simple distillation is a unit operation in which two liquids with different boiling points are separated.
Table of Contents
Principle of simple distillation
Simple distillation is a process of heating and cooling liquids in order to separate and purify them. As the liquid being distilled is heated, the vapors that form are richest in the component of the mixture that boils at the lowest temperature. Purified component boils, and thus turns into vapors, over a relatively small temperature range (2 or 3 °C). A careful observation of the temperature in the distillation flask helps to carry out a good separation. As distillation progresses, the concentration of the lowest boiling component steadily decreases. Eventually, the temperatures within the apparatus begin to change and a pure compound is no longer being distilled. As the temperature continues to increase the boiling point of the next-lowest-boiling compound is approached. When the temperature again stabilizes, another pure fraction of the distillate can be collected. This fraction of distillate is primarily the compound that boils at the second-lowest temperature. This process can be repeated until all the fractions of the original mixture are separated. In order for simple distillation to perform, the two liquids’ boiling points must have a difference of at least 25 °C (or about 77 °F).
Construction of simple distillation
The set of simple distillation consists of a distillation flask with a side arm sloping downwards, Shown in the Figure below. The mouth of the flask is fitted with a cork closure with the inserted thermometer. The condenser is attached to the sloping arm for cold water circulation with inlet at the lower side and an outlet at the upper side. The cold water pipe is attached to inlet while the outlet discharges water to waste. The condenser outlet delivers a liquid product that is collected in a collector or receiver.
Working of simple distillation
- Calibration of thermometer: Calibration can be done by placing the thermometer in an ice bath of distilled water. Allow thermometer to reach thermal equilibrium. Now remove from ice water and place it in a beaker of boiling distilled water and again allow it to reach thermal equilibrium. If the temperatures measured does deviate from the expected values by more than two degrees then use it for recording temperature in distillation process.
- Filling the distillation flask: The flask is filled with not more than two third of its volumes to have sufficient space above the liquid surface so that when boiling begins the liquid will not be propelled into the condenser. This is important in view point of purity of the distillate. Porcelain chips should be placed in the distillation flask to prevent superheating of
the liquid and to cause a more controlled boiling, eliminating the possibility of liquid to bump into the condenser. - Heating the distillation flask: The distillation flask is heated slowly until the liquid begins to boil. The vapours rise up through the neck of the distillation flask and pass throughthe condenser and condense and drip into the collection receiver, Fig. 6.3. Generally rate of distillation is approximately 20 drops per minute. Distillation must occur slowly enough that all the vapours condense to liquid in the condenser. Many organic compounds are flammable and if vapours pass through the condenser without condensing, they may ignite as they come in contact with the heat source.
- Condensation of vapours: As the distillate begins to drop from the condenser, the temperature changes steadily. When it is stable, new receiver is used to collect all the drops that form over a two to three degree range of temperature. As the temperature begins to rise further, a third receiver is used to collect the distillate. This process is repeated; using a new receiver every time the temperature stabilizes or begins changing, until all of the distillate has been collected in discrete fractions. All fractions of the distillate should be saved until it is shown that the desired compound has been effectively separated by distillation.
Handling Precautions
(i) If direct heating is used stop the heat source from the distillation flask before all of the liquid is vaporized.
(ii) When all of the liquid is evaporated, the temperature of the glass of the distillation flask rises very rapidly, possibly ignites whatever vapors still are present in the distillation flask.
(iii) Never distill to dryness. The residue left in the distillation flask may contain peroxides, which could ignite or explode after all the liquid has distilled away.
(iv) Make sure that all joints are secured very tightly. If any vapor escapes at the connection points, it may come into direct contact with the heat source and ignite.
(v) Never heat a closed system, the increasing pressure will cause the glass to explode.
(vi) If the distillation flask has a tapered neck, the thermometer may be placed in such a way as to block the flow of vapors up the neck of the flask; in effect creating a closed system; make sure that if using a tapered neck flask, the thermometer is not resting in the lowest portion of the neck.
(vii) If the liquids comprising the mixture that is being distilled have boiling points closer than 25 °C to one another, the distillate collected will be richer in the more volatile compound but not to the degree necessary for complete separation of the individual compounds.
Advantages
(i) It is simple, cheap, easy and economic method.
(ii) It requires less energy.
(iii) This process requires single run and thus is comparatively faster.
Disadvantages
(i) The final product may contain impurities.
(ii) Azeotropic mixtures cannot be separated by simple distillation.
(iii) Not suitable for mixtures containing thermolabile components.
(iv) The volume of mixture should be not more than 2/3rd of the container.
Applications of simple distillation
(i) Simple distillation is primarily used for the production of distilled water.
(ii) Many volatile oils are separated by simple distillation.
(iii) It is also used in the separation of organic solvents from mixtures.
(iv) It is used to separate non-volatile components from volatile ones.
(v) It is used in preparing pharmaceutical spirits.