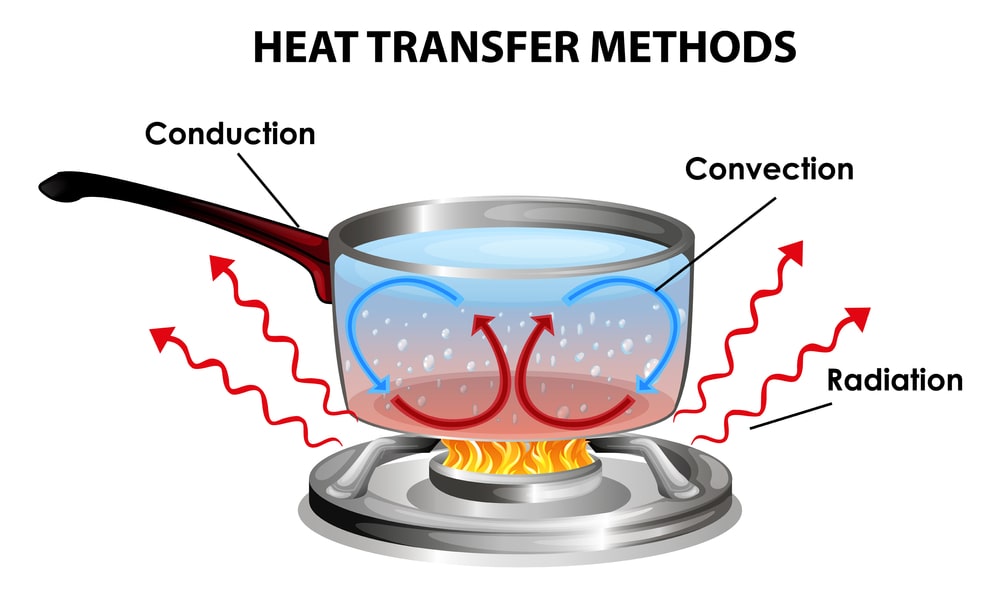
Heat is a form of energy. As we know matter is made-up of atoms and molecules, atoms in molecules are always in motion represented by translation, rotation and vibration. This motion leads to generation of heat energy. The more the motion of atoms in molecules more is the generation of heat. This heat can only be transferred from a region of higher temperature to a region of lower temperature. Heat is transferred by conduction, convection, and radiation. Conduction is the main method by which heat is transferred through solids. In solids no appreciable displacement of matter occurs. The flow of heat in non-metals is due to transfer of vibrational energy from one molecule to another and, in the case of metals, by the movement of free electrons. In convection, energy is transferred from or to a region by the motion of fluids. The heat flow is caused by buoyancy forces (caused by difference in temperature) induced by variations in the density of the fluid. All substances above absolute zero radiate heat in the form of electromagnetic waves. The radiation may be transmitted, reflected, or absorbed by matter; the fraction absorbed being transformed into heat. Thus, it is known that energy is transferred between systems either as heat or as work. The energy transfer that results across a finite temperature difference is heat transfer and the rest of the energy transfer interactions can be brought under one or other type of work transfer. Heat transfers involves first and the second law of thermodynamics.
Objectives of heat transfer
We know that across a finite temperature difference present between a system and its surrounding, heat transfer depends upon the mode of heat transfer for example, conduction, convection or radiation. The amount of heat (q) that transfers depends on several parameters such as the system and surrounding temperatures, their shape, relative movement or flow and thermo-physical properties such as specific heat, thermal conductivity, viscosity, and emissivity. These parameters can be used in understanding the principle and mechanisms involved in heat transfer process which can be further exploited for rapid and economical manufacturing of pharmaceutical API, intermediates and finished dosage forms.
The objectives of heat transfer are given below:
- Formulate basic equation for heat transfers problems.
- Calculate heat transfer between objects with simple geometries.
- Evaluate the impact of initial and boundary conditions on the solutions of a particular heat transfer issue.
- Recognize basic heat transfer mechanism and apply appropriate methods for quantification.
- Evaluate the relative contributions of different modes of heat transfers.
- Perform an energy balance to determine temperature and heat flux.
- Apply heat transfer principles to design and size to evaluate performance of heat exchangers.
Applications of heat transfer
- Evaporation: Heat is supplied in order to convert a liquid into a vapour.
- Distillation: Heat is supplied to the liquid mixture for separation of individual vapour component.
- Drying: For drying the wet granules and other solids.
- Crystallization: Saturated solution is heated to bring out super saturation, which promotes crystallization of drugs.
- Sterilization: Autoclaves are used with steam as a heating medium.
- Food processing industries: The principles of heat transfers are widely used in foodprocessing industries for pasteurization, refrigeration, chilling, and freezing and refrigerative evaporation.
- Chemical process industries: Heat transfers methods find a variety of applications in the chemical process industries. Heating and cooling of batch tanks will allow the user to calculate the time it takes to heat up and then cool a batch vessel or tank.
- Manufacturing of bulk drugs and dosage forms: Principles of heat transfer is of significance in manufacturing of various bulk drugs, excipients and dosage form.
Make sure Check our Amazing article on: Bag Filter