Positive rays/anode ray: Eugen Goldstein utilized a cathode discharge tube with a hole in 1886. While cathode rays were flowing away from the cathode, he noticed that colored rays were being created at the same time, passing through the perforated cathode and causing a light on the wall opposite the anode. Thomson investigated these rays and discovered that they were made up of particles with a positive charge. Anode ray, he termed them.
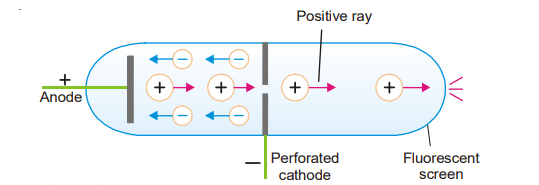
Table of Contents
Properties of Anode ray
(1) They move in the opposite direction of the cathode in a straight line.
(2) They are positively charged because they are deflected by electric as well as magnetic fields.
(3) The charge-to-mass ratio (e/m) of positive particles differs depending on the kind of gas used in the discharge tube.
(4) They have a mass that is several times that of an electron.
(5) They induce zinc sulphide to glow.
How are Anode ray produced?
When high-speed electrons (cathode rays) strike a molecule of a gas placed in the discharge tube, they knock out one or more electrons from it. Thus a positive ion results
M+e– ——- M++ 2e–
These positive ions pass through the perforated cathode and appear as an Anode ray. When an electric discharge is passed through the gas under high electric pressure, its molecules are dissociated into atoms and the positive atoms (ions) constitute the positive rays.
Conclusions from the study of Positive rays
Thomson and Aston (1913) inferred from a study of the characteristics of positive rays that an atom consists of at least two parts: (a) electrons; and (b) a positive residue with which the atom’s mass is connected.
Make sure check our amazing article : Determination of the charge on electron