Primary emulsion: The emulsion is prepared by taking oil, water, and gum, with rapid trituration until a clicking sound is produced and the product becomes white. At this stage, the emulsion is called primary emulsion. it can be prepared either by dry gum method or wet gum method.
Table of Contents
Types of emulsion and definition
An Emulsion is a biphasic liquid preparation containing two immiscible liquids, one of which is dispersed as minute globules into the other
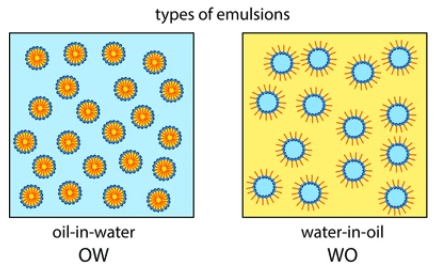
The Emulsions are classified into 2 types:
- Oil in water type (o/w)
- Water in oil type (w/o)
a. Oil in water type Emulsion: Oil is in the dispersed phase, whereas water is in the continuous phase. These are preferred for Internal use
b. Water in oil type (w/o): Water is in dispersed phase, oil is in the continuous phase. These are mainly used Externally as lotions (or) creams.
Define creaming and cracking
Creaming may be defined as the upward movement of dispersed globules to form a thick layer at the surface of the emulsion.
Cracking means the separation of two layers of dispersed and continuous phase, due to the coalescence of dispersed phase globules which are difficult to redisperse by shaking.
What is H.L.B value
- Griffin devised a useful method for calculating balanced mixtures.
- To provide a particular type of emulsion.
- It is called hydrophile lipophile balance [HLB].
- It is given a number on the HLB scale, which is divided into 18 units.
- An emulsifying agent with higher numbers (8-18) indicates hydrophilic properties and produces o/w type emulsion, with lower numbers (3-6) representing lipophilic properties and producing w/o type emulsion.
Ex: H.L.B value of Acacia – 8 H.L.B value of SLS – 40.
What is stoke’s law
Strokes law V=2r2 (d1-d2)g/9 η
V = Rate of creaming
r = Radius of globules
d1 = Density of dispersed phase
d2 = Density of continuous phase
g= (gravity) gravitational constant
η=Viscosity of dispersion medium
What is phase inversion
It means the change of one type of emulsion into another type of emulsion.
It means:
- o/w type emulsion changes into w/o type emulsion and
- w/o type emulsion changes into o/w type emulsion
Reasons:
- Addition of electrolytes
- Changes of temperature
- Changing of emulsifying agent
- Changes of ratios
Also read: Identification test for emulsion