Table of Contents
Rate of drying
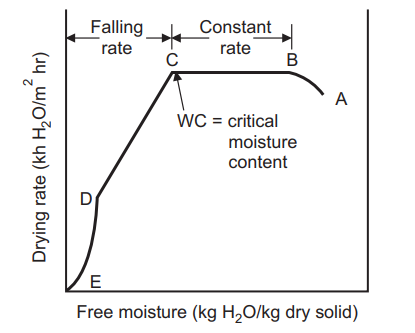
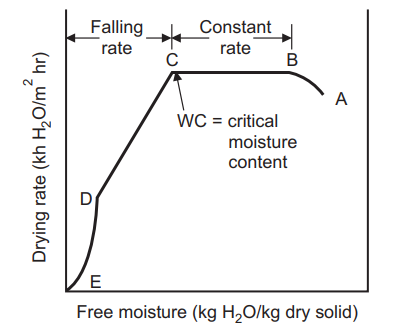
When a wet non-porous granular solid material is placed in a tray and dried with a carrier gas like air under constant drying conditions of velocity, temperature, humidity, and pressure of air at the inlet, the experimental data can be plotted as drying rate vs. free moisture content or time, Shown in the figure below. The obtained typical drying rate curve is divided into a constant rate period (AB), a first falling rate period (BC), and a second falling rate period (CD). The free moisture content at the end of the constant rate period (B) is known as CMC. During the drying process, the moisture content is plotted based on the entire material. In general, at the start of the drying process, there is an unsteady state of the warming-up period during which wet solid keep changing till the beginning of the constant rate period.
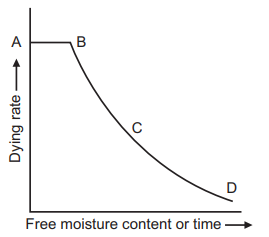
The drying rate curves are not smooth and continuous which indicates that the drying process involves a single mechanism throughout. In drying calculations, the water content in the wet solid is usually expressed on a dry weight basis, i.e. Kg of water/Kg of dry solid.
Applications of Moisture Content Determination
Drying curve
For each and every product, there is a representative curve that describes the drying characteristics for that product at a specific temperature, velocity, and pressure conditions. This curve is referred to as the drying curve for a specific product. Variations in the curve will occur principally in rate relative to carrier velocity and temperature. The curve is extremely valuable in understanding unusual behavior associated with the drying of each unique product. The drying process can be divided into three periods:
(a) Constant drying rate period.
(b) First falling drying rate period.
(c) Second falling rate period.
(a) Constant Drying Rate Period
In a constant period, material or mass of material contain much of water that liquid surface exists which dries in a similar fashion to an open-faced body of water. Diffusion of moisture from within the droplet maintains saturated surface conditions and as long as this lasts, evaporation takes place at constant rate. When a solid is dried under constant drying conditions, the moisture content (MC) typically falls. The graph is linear at first, then curves and eventually levels off. Constant rate drying period (B-C) will proceed until free moisture appears from the surface, the moisture removal rate will then become progressively less. At CMC the drying rate ceases and remains constant. During the constant rate period, the moisture from the interior migrates to the surface by various means and is vaporized.
As the moisture content is lowered, the rate of migration to the surface is also lowered. If drying occurs at too high temperatures, the surface forms closely packed shrunken cells which are sealed together. This acts as a barrier to moisture migration and tends to keep the moisture sealed within. This condition is known as ‘case hardening’. The constant rate period is characterized by a drying independent of moisture content. During this period, the solid is so wet that a continuous film of water remains over the entire drying surface, and this water acts to lower the drying rate. The temperature of the wetted surface attains the wet-bulb temperature.
Web Bulb Temperature (WBT): WBT is the steady-state temperature shown by the thermometer whose bulb is covered with a wet wick and from which water is evaporating into a high-velocity air stream. The quantity of water evaporated is not high enough to alter the temperature and humidity of the air stream. The air is blown at high velocity (minimum 300 m/min) to cause evaporation of water from the wick. Evaporation requires latent heat. This heat comes from the surface of the glass bulb of the thermometer. So the temperature of the glass bulb decreases. The heat comes from the temperature difference between Tw and Ta (large). It is the case of simultaneous heat and mass transfer. This heat is latent heat for the phase change of water to water vapor.
(b) Falling Rate Periods
The constant rate period ends when the migration rate of water from the interior of the surface becomes less than the rate of evaporation from the surface. The period subsequent to the critical point is called ‘the falling rate period’. Following this point, the surface temperature rises, and the drying rate falls off rapidly. The falling rate period takes a far longer time than the constant rate period, even though the moisture removal may be much less. The drying rate approaches zero at some equilibrium moisture content. Drying in falling rate period involves two processes:
(a) Movement of moisture within the material to the surface.
(b) Removal of the moisture from the surface.
The method used to estimate drying rates and drying times in the falling rate period depends on whether the solid is porous or non-porous. In a non-porous material, once there is no superficial moisture, further, drying can occur only at a rate governed by diffusion of bulk moisture to the surface. In a porous material, other mechanism appears, and drying takes place in the bulk of the solid instead of at the surface.
(i) First falling period
The moisture content at the end of the constant rate period (point c), is the ‘critical moisture content’. At this point, the surface of the solid is no longer saturated, and the rate of drying decreases with the decrease in moisture content. At point C, the surface moisture film evaporates fully, and with the further decrease in moisture content, the drying rate is
controlled by the rate of moisture movement through the solid.
(ii) Second falling period:
Period C to D represents conditions when the drying rate is largely independent of conditions outside the solid. The moisture transfer may be due to any combination of liquid diffusion, capillary movement, and vapor diffusion.
Effect of Shrinkage:
A factor that often greatly affects the drying rate is the shrinkage of the solid as moisture is removed. Rigid solids do not shrink appreciably, but colloidal and fibrous materials do undergo shrinkage. The most serious effect is the development of a hard layer on the surface that is impervious to the flow of liquid or vapor moisture and slows down the drying rate. In many materials, if drying occurs at too high a temperature, a layer of closely packed, shrunken cells, which are sealed together, forms at the surface that presents a barrier to moisture migration. Another effect of shrinkage is to cause the materials to warp and change their structure. Sometimes, to decrease these effects of shrinkage, it is desirable to dry with moist air. This decreases the rate of drying so that the effects of shrinkage on warping or hardening at the surface are greatly reduced.