Table of Contents
Pharmaceutics-1 B pharmacy QB SEM 1(by Rguhs)
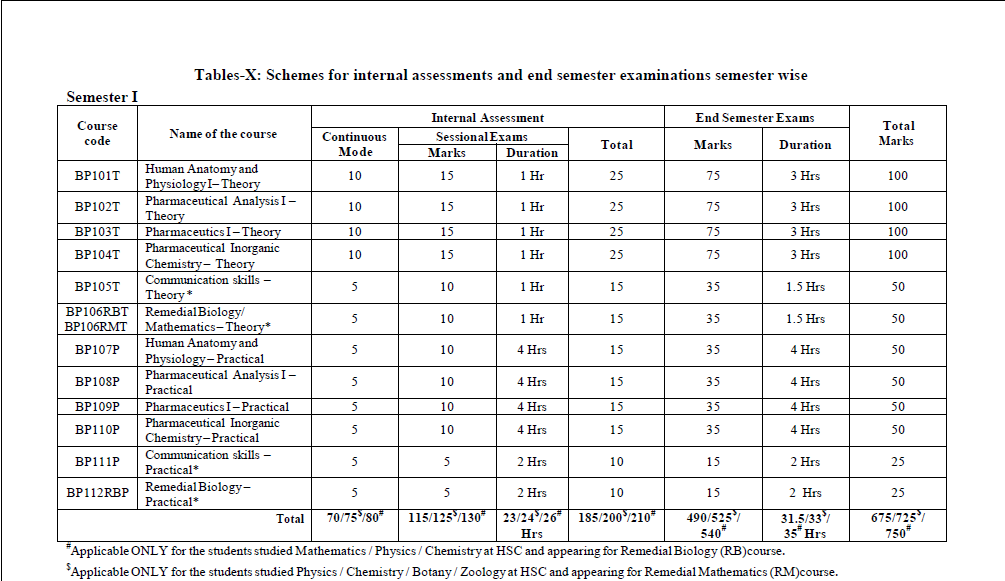
UNIT-1 Pharmaceutics question bank
LONG ESSAY (10M) Pharmaceutics question bank
- Discuss in detail the historical background and development of pharmacy in India.
- Define prescription. with the help of an ideal example describe the importance of all the parts of a prescription.
- Define prescription. explain the handling of prescriptions. write about the sources of errors in prescription.
- explain the factors affecting dose selection. give any two formulae to calculate children’s dose.
- Define posology. enumerate different factors affecting the selection of the dose of a drug
SHORT ESSAY (5 Marks)
- Discuss the brief historical background of the profession of pharmacy in India.
- Significance of the profession of pharmacy in relation to education and industry.
- Write a note on pharmacy as a career.
- Classify monophasic liquid dosage forms with examples.
- Define dosage form and classify with examples.
- Discuss Pediatric dose calculations based on age, body weight, and body surface area.
- Define isotonicity. Write any two formulas to adjust the isotonicity.
- What will be the dose for a child of 5 years if the adult dose of a drug is 400mg?
- Calculate the dose for a child that has a body surface area of 0.57m, when the adult dose of a drug is 100mg.
SHORT ANSWERS (2 Marks)
1.What is Pharmacopoeia? Mention all the editions of Indian Pharmacopoeia.
2. Give the significance of Pharmacopoeias.
3. Enlist various Pharmacopoeias.
4. List the editions of Indian Pharmacopoeia chronologically
5. Mention the contents of the National Formulary of India.
6. Differentiate between Indian Pharmacopoeia and National Formulary of
India.
7.What is the latest edition and year of publication of the Indian
Pharmacopoeia?
8. Write the difference between Pharmacopoeia and Formulary.
9. Write any four salient features of the first edition of Indian Pharmacopoeia.
10. Write any four salient features of the second edition of Indian Pharmacopoeia.
11. Write any four salient features of the third edition of Indian Pharmacopoeia.
12. Write any four salient features of the fourth edition of Indian Pharmacopoeia.
13. Define monophasic liquid dosage forms with examples.
14. Names any four monophasic dosage forms used externally for example.
15 Names any four monophasic dosage forms used internally with example.
16. Define the terms Synergism’ and Idiosyncrasy’.
UNIT- II Pharmaceutics question bank
SHORT ESSAY (5 Marks)
1. Discuss briefly solubility enhancement techniques.
2. Define powders. Classify powders.
3. Explain geometric dilution with an example.
4. Discuss the different methods of mixing powders.
5. Explain simple and compound powders with an example.
6. How do you prepare effervescent granules by the fusion method?
7. How do you dispense eutectic powders?
8. Explain insufflations with examples.
9. Write the advantages and disadvantages of powders as the dosage form.
10. Define and classify powders based on the official grades of powders.
11. Explain dusting powders with examples.
12. Define preservatives. Classify with examples.
13. Define stabilizers. Explain with examples.
14. Explain the organoleptic additives used in monophasic liquid dosage forms with examples.
15. Explain in detail the different vehicles used in monophasic dosage forms.
Give their advantages and disadvantages.
SHORT ANSWER (2 Marks)
- Enlist different solubility enhancement techniques.
- Names any four solvents used in the preparation of monophasic liquid dosage forms.
- Names any two antioxidants used in liquid formulations.
- Write any two examples for colouring agents and flavouring agents used in monophasic dosage forms.
- Name any two examples of stabilizers used in monophasic liquid dosage forms.
- Name any two antioxidants and preservatives used in monophasic liquid dosage forms.
- Define antioxidants with examples.
- Define preservatives with examples
- Define stabilizers with examples.
- Give the metric equivalents for the following: (a) one grain, (b) one ounce, (c) one teaspoonful, (d) one tablespoonful.
- Give the metric equivalents for the following: (a) one minim, (b) one fluid ounce, (c) one tumblerful, (d) one quart.
- Give the metric equivalents for the following: (a) one cup, (b) one pound, (c)one drop, (d) one wineglassful.
- How many grams of a drug are required to make 120ml of a 25%w/v solution?
- What is the percentage strength (%w/v) of a solution containing 450 mg of medicament dissolved in 90 ml of a solvent?
- How much potassium permanganate would be required to prepare 50 ml of potassium permanganate solution of 2.8%w/v strength?
- In what ratio 90 % alcohol and 30% be mixed to give 60% alcohol?
- How many grams of dextrose are required to prepare 900ml of 10%w/v solution?
- How many parts of 15%, 10% and 5% alcohols are mixed to prepare 8% alcohol?
- How do you prepare 1 litre of 5% w/v dextrose solution from 50% w/v dextrose solution?
- How do you prepare 500 ml of 50% alcohol from 90% alcohol?
- How do you prepare of 50% alcohol from 80% alcohol and 30% alcohol?
- How many litres of 8% solution can be prepared from 500gm of a solid?
- What are isotonic solutions?
- Define isotonic and paratonic solutions.
- Define ‘allegation’ and ‘proof spirit’.
- What is the proof strength of 45% v/v alcohol?
- Find the strength of 90% v/v alcohol in terms of proof spirit.
- Convert 90% v/v and 40% v/v alcohol in to proof strength.
- Convert 40% v/v alcohol in to proof spirit.
- How do you prepare 50 litres of proof spirit from 90% v/v alcohol?
- What is the proof spirit of an elixir containing 42%. alcohol?
- What is the proof spirit of a 1% v/v alcohol?
- Define the terms ‘proof spirit’ and ‘isotonicity’.
- Calculate the actual strength of 25° O.P. (overproof).
- Calculate the actual strength of 45° U.P. (under proof. o.P
- What are hypertonic and hypotonic solutions?
- Calculate the percentage of sodium chloride required to render a procaine
HCI iso-osmotic with blood plasma. (1% w/v solution of procaine HCl has a freezing- point of 0.122oC and 1% w/v sodium chloride has a freezing point of 0.576°C)
38. Define hygroscopic and deliquescent powders.
39. How do you dispense potent powders?
40. Why is a double wrapping of powder required?
41. Classify powders.
42. Define cachets for example.
43. Define powder with an example.
44. Define and classify dusting powders.
45 Define eutectic powders.
46, Define insufflations with examples.
47. Define simple and compound powders.
48. What are the ingredients of dusting powders?
49. Define geometric dilution.
50. Enlist the methods of mixing powders.
UNIT-III Pharmaceutics question bank
LONG ESSAY (10 marks)
- Discuss briefly the stability problems and methods to overcome the suspension.
- Define suspension. Explain the preparation of suspension containing diffusible and diffusible solids?
- Define suspension. Write its advantages, disadvantages s and classification
suspensions. Differentiate flocculated and deflocculated suspension?
- What is the various instability of emulsion? Discuss them with their cause and precautions to avoid them?
- Define and classify emulsion. Write the various identification tests for emulsion type?
SHORT ESSAYS (5 Marks)
- Define and classify suspension. Write the advantages and disadvantages of suspension?
- Differentiate flocculated and deflocculated suspension.
- Discuss briefly the method of preparation of suspensions containing indiffusible solids.
- Differentiate mouth washes and Gargles.
- Differentiate lotions and liniments.
- Explain controlled Nocculation?
- Discuss various methods of preparation of cmulsions.
- Write the principle and procedure involved in the preparation of syrup 1.P.
- Differentiate between elixirs and syrups.
- Write a note on identification tests for emulsions with example.
- Write a note on why emulsions arc white to creamy white.
SHORT ANSWERS (2 Marks)
- Define gargle with examples.
- Define mouthwashes with examples. o and 2-
- Define ear drops and nasal drops for example.
- Write the advantages of syrups.
- What is invert sugar?
- Define linctuses with examples
- Define expectorant with examples.
- Define throat paint with examples.
- Define elixirs with examples.
- Define enema with examples.
- Deine nasal drops with examples.
- What are structured vehicles? Give examples.
- Name any two suspending and emulsifying agents.
- Name any four flocculating agents used in the preparation of suspension.
- Name any two flocculating and deflocculating agents.
- What is the phase volume ratio? How it is useful in the preparation of emulsions.
- What is phase inversion? How it can be prevented.
- Classify emulsifying agents.
- Write the primary emulsion formula for fixed oils and mineral oils.
- Write the primary emulsion formula for oleoresin and volatile oils.
- Write the primary emulsion formula for fixed oils and volatile oils.
- Classify emulsions.
- Classify suspensions.
- Why emulsifying agent is required in the preparation of emulsions.
- Define creaming and cracking?
- Give Griffin’s HLB value scale and its application.
- What should be the HLB of emulsifying agent to give oil in water or water in oil emulsions?
- Give two examples for wetting agents.
- Define the wetting phenomenon.
- Define surfactants with examples.
- Enlist various identification tests for emulsion.
- How do differentiate monophasic and biphasic liquid dosage forms for example?