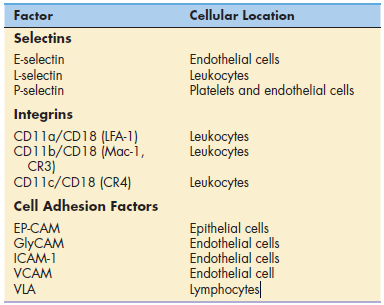
Tissue damage, infection, or inflammation cause the migration
of white blood cells from the blood vessel to tissue through a
process called diapedesis or lymphocyte diapedesis. Within 2 hours of the initiation of
an inflammatory response, small-molecular-weight proteins,
called cytokines, are released by monocytes. Cytokines upregulate
adhesion molecules, called E-selectin and P-selectin, on vessel walls. Other molecules, called chemokines, also are
released by endothelial cells.
Chemokines attach to endothelial cells along a concentration
gradient that is counter to blood flow (the highest concentration
is at the site of diapedesis). In essence, chemokines
attract leukocytes to the site of infection or tissue damage.
However, leukocytes move through blood vessels at great
speed and must be slowed and tethered to the vessel walls
before they exit the vessel and enter the tissue.
To slow the speed of leukocytes, E-selectins, and chemokines
interact with carbohydrate ligands on leukocytes. Different selectin–ligand interactions help
slow-rolling PMNs and lymphocytes. PMNs are slowed by interactions between a tetrasaccharide carbohydrate present
on PMNs and monocytes called sialyl-Lewisx and E-selectins.
Lymphocytes roll faster than monocytes or PMNs and require
interactions among E-selectin, P-selectin, and lymphocyte
VLA-4 (very late antigen 4) in the deceleration process.
Used in Lymphocyte diapedesis
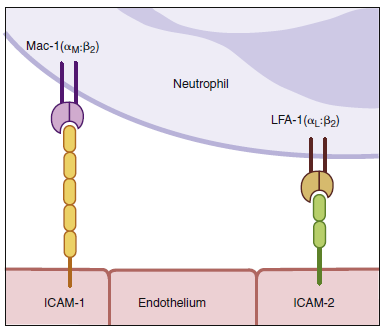
To tether leukocytes to the vessel walls, additional interactions
between endothelial intercellular adhesion molecules
(ICAM-1 and ICAM-2) and lymphocyte function-associated
antigen -1 (LFA-1) are required. Once the
cells are tethered to the endothelium, they flatten out and transmigrate through the endothelial layer and the underlying matrix.
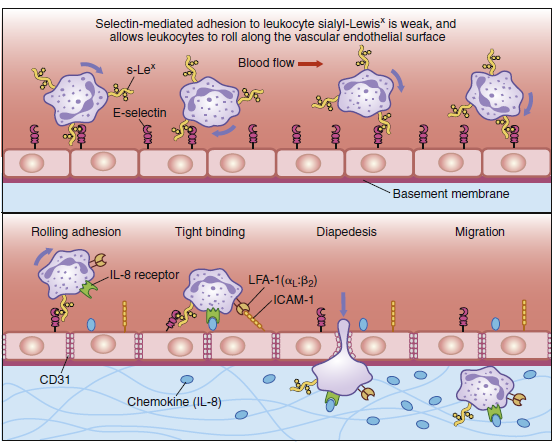
Diapedesis of neutrophils. Neutrophils leave the blood and migrate to sites of infection in a multiple-step
process mediated through adhesive interactions that are regulated by macrophage-derived cytokines and
chemokines. The first step (top panel) involves the reversible binding of leukocytes to the vascular endothelium
through interactions between selectins induced on the endothelium and their carbohydrate ligands
on the leukocyte, shown here for E-selectin and its ligand the sialyl-Lewisx moiety (s-Lex). This interaction
cannot anchor the cells against the shearing force of the flow of blood; and, instead, they roll along
the endothelium, continually making and breaking contact. The binding does, however, allow stronger
interactions, which occur as a result of the induction of intracellular adhesion molecule 1 (ICAM-1) on the
endothelium and the activation of its receptors LFA-1 and Mac-1 (not shown) on the leukocyte by contact
with a chemokine like an interleukin 8 (IL-8). Tight binding between these molecules arrests the rolling and
allows the leukocyte to squeeze between the endothelial cells forming the wall of the blood vessel (to
extravasate). The leukocyte integrins LFA-1 and Mac-1 are required for extravasation and for migration
toward chemoattractants. Adhesion between molecules of CD31, expressed on both the leukocyte and the
junction of the endothelial cells, is also thought to contribute to extravasation. The leukocyte also needs to
traverse the basement membrane; it penetrates this with the aid of a matrix metallo-proteinase enzyme that
it expresses at the cell surface. Finally, the leukocyte migrates along a concentration gradient of chemokines
(here shown as IL-8) secreted by cells at the site of infection.
The tethering or adherence process may take only seconds,
but the transmigration of leukocytes may take 10 to
20 minutes.
During transmigration, immunocompetent cells
change from around the structure to a flat structure and crawl to
the exit site. Cells can exit the vasculature by passing between
or through endothelial cells. The passage between cells is facilitated
by the production of hevin, which temporarily disrupts
cell junctions and allows cell movement between cell junctions.
Migration through cells is a more complex process. Following
attachment, the lymphoid cells are concentrated in the
caveolin-rich areas on the cell surface, which are called transmigratory
cups. Caveolins are a family of proteins that mediate
the endocytosis of receptor-bound molecules. Following
the endocytosis of leukocytes, ICAMs and caveolins form a
protective channel that allows the leukocyte passage through
the cell. The formation of another transmigratory cup allows
the leukocyte to exit both the vascular endothelial cell and the
underlying matrix.