Equilibrium moisture content: The moisture contained in a material comprises all those substances which vaporize on heating and lead to a weight loss of the sample. The weight is determined by a balance and interpreted as the moisture content. As per this definition, moisture content includes not only water but also other mass losses such as evaporating organic solvents, alcohols, greases, oils, aromatic components, as well as decomposition and combustion products. The moisture content is also called moisture assays which is one of the most important analyses performed on most pharmaceutical products. Water activity measurements parallel to the moisture content are also an important parameter for the quality and stability of pharmaceuticals.
The moisture in products can be present in different forms based upon the type of bonding with solids, Showing in the Figure below. It is called “free water” when water is on the surface of the test substance and it retains its physical form, “absorbed water” when water is present in large pores, cavities, or capillaries of the test substance, and “water of hydration” occluded in lattice ions or water of crystallization coordinately bonded to ions.
The loss on drying (LOD) is the amount of water and volatile matters present in a sample when the sample is dried under specified conditions. Moisture content (MC) is the quantity of water contained in a material, such as raw materials, API, and blend.
\%\;Loss\;of\;drying\;=\;\frac{Mass\;of\;water\;in\;sample\;(kg)}{Total\;mass\;of\;wet\;sample\;(kg)}\times\;100 \%\;Moisture\;content\;=\;\frac{Mass\;of\;water\;in\;sample\;(kg)}{Mass\;of\;dry\;sample\;(kg)}\times\;100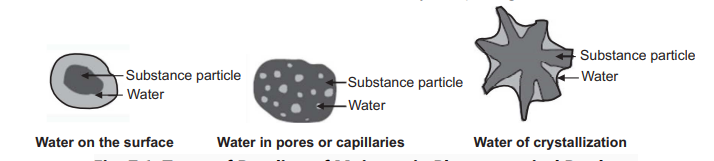
The moisture content of a solid in excess of the equilibrium moisture content is referred to as free moisture (water). It must be noted that during drying, only free moisture is evaporated. The free moisture content (FMC) of a solid depends upon the vapor concentration in the air above the solid surface. The moisture content of a solid when it is in equilibrium with a given partial pressure of vapor in the gas phase is called equilibrium moisture content (EMC). Similarly, the moisture content at which the constant rate drying period ends and the falling rate drying period starts is called the critical moisture content (CMC). During the constant rate drying period, the moisture evaporated per unit time per unit area of drying surface remains constant, and in the falling rate drying period, the amount of moisture evaporated per unit time per unit area of drying surface continuously decreases.
When the water vapour pressure of the air approaches the saturation water vapour pressure at the temperature of the gas, the EMC of materials rapidly increases. At these stages, the process undergone by the material is not only adsorption. Water vapour begins to condense within the pore structures of the materials. Theoretically, if the material is in contact with air that is 100 % saturated for a very long period, all pores of the material should be filled with condensed moisture. The EMC that corresponds to that hypothetical state is called the saturation moisture content (SMC) of the material. But in practice, the rate of this process becomes infinitesimally small at an EMC that is known as the capillary saturation moisture content (CSMC) and is often substantially less than the saturation moisture content referred to above.
Table of Contents
Measurements of Equilibrium moisture content
The moisture content is determined by several direct and indirect methods.
(i) Direct Methods
The direct methods include mainly thermogravimetric methods. The moisture content can be determined by an oven method directly. The solid is weighed and dried, then weighed again according to standardized procedures. In the Thermogravimetric method, moisture is always separated. Thus, there is no distinction made between water and other readily volatile product components. A representative sample must be obtained to provide a useful moisture content evaluation. Also, the moisture content of the product must be maintained from the time the sample is obtained until the determination is made by storing it in a sealed container. Thermogravimetric techniques can be used to continuously measure the mass of a sample as it is heated at a controlled rate. The temperature at which water evaporates depends on its molecular environment. The free water normally evaporates at a lower temperature than bound water. Thus by measuring the change in the mass of a sample as it loses water during heating it is often possible to obtain an indication of the amounts of water present in different molecular environments. For many solids, this method is mandatory, particularly for granules. For granules, the moisture content is measured by heating them in a hot air oven at a suitable temperature until the weight becomes constant. For heat-sensitive materials vacuum is applied in the oven to decrease the boiling point of the liquid.
(ii) Indirect Methods
Indirect methods are developed to determine the moisture content rapidly. For example, the use of modern heating measurement methods like infrared, microwaves, ultrasound, and spectroscopy. These methods are developed due to requirements of rapid, non-destructive, and precise moisture content determination. The indirect methods are generally faster than the direct methods for moisture determination. When done properly, the indirect methods can be accurate and precise. However, the accuracy and precision of the indirect methods depend on careful preparation and analysis of known standards to establish reliable calibration curves. Indirect methods require a large capital investment in equipment. Nevertheless, preparation of the standards and accurate calibration curves must be verified by a specific direct method to establish a reliable indirect method of instrumentation that can achieve accurate and precise predicted values.
The methods for moisture determination given in USP24 NF19 are the best, classical, and addresses only the determination of moisture content. The U.S.P. offers two methods for the determination of moisture content in solids:
(a) Titrimetry (Karl Fisher titration).
(b) Gravimetric (Thermal gravimetric analysis).
Moisture content is used in a wide range of scientific and technical areas and is expressed as a ratio, which can range from 0 (completely dry) to the value of the material’s porosity at saturation. It can be given on a volumetric or mass (gravimetric) basis. Moisture content is expressed as a percentage of moisture based on the total weight (wet basis) or dry matter (dry basis). Wet basis moisture content is generally used. The moisture content is expressed by following formulae.

Based on the different forms of moisture present in the material the method used for the measurement of moisture may estimate more or less moisture content. Therefore, for different pharmaceutical products, Official Methods of moisture measurement have been given by agencies.
Read too: What is drying
