Solubility is the ability of one substance to fully dissolve in another substance under specified conditions. The word soluble comes from the fourteenth century, from the Latin word ‘solvere’ meaning to dissolve.
The concentration of a solution is usually quoted in terms of the mass of the solute dissolved in a particular volume of solvent. Solubility is generally expressed in grams per liter. Therefore, the solubility of a solute in a solvent at a particular temperature is the number of grams of the solute necessary to saturate 100 grams or mL of the solvent at that temperature. The most commonly encountered solutions are solids dissolved in liquids. The solid that dissolves in a liquid is the solute, and the liquid in which it dissolves is the solvent. A solute is the dissolved agent, usually the less abundant part of the solution, whereas a solvent is the more abundant part of the solution. If a solid can dissolve in a liquid, it is said to be soluble in that liquid, if not, it is said to be insoluble. As we add more solids to a liquid, the solution becomes more concentrated.
The greater the solubility of a substance, the more concentrated it is possible to make the solution. Solubility is measured after the solute of interest has had sufficient contact time (however long it takes) with the solvent. There are two types of solubility: one is called intrinsic solubility, and the other is apparent solubility. Intrinsic solubility is defined as the maximum concentration to which a solution can be prepared with a specific solute and solvent. It is often derived from calculations and is a single numeric number (for example, 0.5 µg/mL) that is independent of environmental factors. The apparent solubility is dependent on environmental factors such as pH and ionic strength and is obtained from the experimental measurements. The rate of solubility is affected by many factors, such as the type of solvent, size and amount of solute particles, stirring speed, and temperature. The concept of solubility is very important because it governs the preparation of solutions as dosage forms and a drug must be in solution before it can be absorbed by the body or have any biological activity. Since the activity of the drug depends on solubility, it is equally important to control environmental conditions that impact various types of solutions.
Table of Contents
Solubility expressions
The solubility of a drug or other substance in a solvent can be expressed quantitatively in numerous terms, viz. percent by mass, percent by volume, molality (m), molarity (M), mole fraction (x), parts per million (ppm), etc. The particular terminology we use depends largely on the use to which we will put it. The solubility of a substance is defined as the amount of solute dissolved in a specific amount of solvent at a specific temperature. The British Pharmacopoeia and other official chemical and pharmaceutical compendia frequently use the term parts per part of solvent (for example, parts per million, ppm). The expressions ‘insoluble’, ‘very highly soluble’, and ‘soluble’ also be used to express the solubility of solutes, but they are inaccurate and often not found to be helpful. For quantitative work, specific concentration terms must be used. Most substances have at least some degree of solubility in water, and while they may appear to be ‘insoluble’ by a qualitative test, their solubility can be measured and quoted precisely. In aqueous media at pH 10, chlorpromazine base has a solubility of 8 × 10-6 mol/dm3. It is very slightly soluble, and it might be considered as ‘insoluble’ upon visual inspection due to the lack of disappearance of solids.
In many solutions, the concentration has a maximum limit that depends on various factors, such as temperature, pressure, and the nature of the solvent. Relative concentrations of a solute/solvent system can often be expressed by the terms dilute and concentrated, or by the terms unsaturated, saturated, and supersaturated. Solutes in water are often categorized as either strong electrolytes, if completely ionized, weak electrolytes, if only partially ionized or non-electrolytes, when non-ionized. In regard to solubility, general terms can be used when describing whether a compound is soluble or not. These terms are given in Table 1.1, and are based on the part of solvent needed to dissolve 1 part of the solute for example, testosterone is considered insoluble in water but soluble in alcohol, ether, or other organic solvents. Fortunately, when injected into the body, insoluble testosterone is diluted, and the larger volume of body water permits testosterone to go into the solution.
| Term | Parts of solvent required per part of solute |
| Very soluble | Less than 1 part |
| Freely soluble | 1 – 10 |
| Soluble | 10–30 |
| Sparingly soluble | 30–100 |
| Slightly soluble | 100 – 1000 |
| Very insoluble | 1000 – 10,000 |
| Insoluble | More than 10,000 |
Saturated Solution
A solution in which the dissolved solute is in equilibrium with the undissolved solute or solid phase is known as a saturated solution. It is when no more of the solid will dissolve into the solution. When we add a solute to a solvent, a point is reached where no more solute dissolves under specified conditions. The solution is saturated. The concentration of the solute in a saturated solution is the solubility of the solute in that solvent at that temperature. Saturation of solution can also be defined as the point where the solution is in equilibrium with undissolved solute. In a saturated solution containing an undissolved solid solute, the rate at which the molecules or ions leave the solid surface is equal to the rate at which the solvated molecules return to the solid.
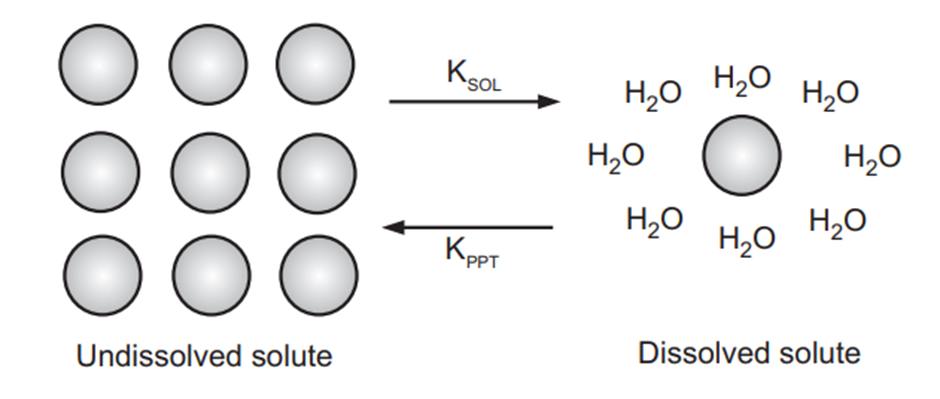
In Fig. 1.1, KSOL is the rate constant at which the solid is solvated, and KPPT is the rate constant at which the solvated molecule is returned to the solid. The solubility of a substance is the ratio of these rate constants at equilibrium in a given solution. At equilibrium, the rate at which a solute precipitates out of the solution is equal to the rate at which the solute goes into the solution.
Solubility is the ability of one substance to fully dissolve in another substance under specified conditions. The word soluble comes from the fourteenth century, from the Latin word ‘solvere’ meaning to dissolve.
The concentration of a solution is usually quoted in terms of the mass of the solute dissolved in a particular volume of solvent. Solubility is generally expressed in grams per liter. Therefore, the solubility of a solute in a solvent at a particular temperature is the number of grams of the solute necessary to saturate 100 grams or mL of the solvent at that temperature. The most commonly encountered solutions are solids dissolved in liquids. The solid that dissolves in a liquid is the solute, and the liquid in which it dissolves is the solvent. A solute is the dissolved agent, usually the less abundant part of the solution, whereas a solvent is the more abundant part of the solution. If a solid can dissolve in a liquid, it is said to be soluble in that liquid, if not, it is said to be insoluble. As we add more solids to a liquid, the solution becomes more concentrated.
The greater the solubility of a substance, the more concentrated it is possible to make the solution. Solubility is measured after the solute of interest has had sufficient contact time (however long it takes) with the solvent. There are two types of solubility: one is called intrinsic solubility, and the other is apparent solubility. Intrinsic solubility is defined as the maximum concentration at which a solution can be prepared with a specific solute and solvent. It is often derived from calculations and is a single numeric number (for example, 0.5 µg/mL) that is independent of environmental factors. The apparent solubility is dependent on environmental factors such as pH and ionic strength and is obtained from the experimental measurements. The rate of solubility is affected by many factors, such as type of solvent, size and amount of solute particles, stirring speed, and temperature. The concept of solubility is very important because it governs the preparation of solutions as dosage forms and a drug must be in solution before it can be absorbed by the body or have any biological activity. Since the activity of the drug depends on solubility, it is equally important to control environmental conditions that impact various types of solutions.
Solubility expressions
The solubility of a drug or other substance in a solvent can be expressed quantitatively in numerous terms, viz. percent by mass, percent by volume, molality (m), molarity (M), mole fraction (x), parts per million (ppm), etc. The particular terminology we use depends largely on the use to which we will put it. The solubility of a substance is defined as the amount of solute dissolved in a specific amount of solvent at a specific temperature. The British Pharmacopoeia and other official chemical and pharmaceutical compendia frequently use the term parts per part of solvent (for example, parts per million, ppm). The expressions ‘insoluble’, ‘very highly soluble’, and ‘soluble’ also be used to express the solubility of solutes, but are inaccurate and often not found to be helpful. For quantitative work, specific concentration terms must be used. Most substances have at least some degree of solubility in water, and while they may appear to be ‘insoluble’ by a qualitative test, their solubility can be measured and quoted precisely. In aqueous media at pH 10, chlorpromazine base has a solubility of 8 × 10-6 mol/dm3. It is very slightly soluble, and it might be considered as ‘insoluble’ upon visual inspection due to the lack of disappearance of solids.
In many solutions, the concentration has a maximum limit that depends on various factors, such as temperature, pressure, and the nature of the solvent. Relative concentrations of a solute/solvent system can often be expressed by the terms dilute and concentrated, or by the terms unsaturated, saturated, and supersaturated. Solutes in water are often categorized as either strong electrolytes, if completely ionized in water, weak electrolytes, if only partially ionized, or non-electrolytes, when non-ionized. In regard to solubility, general terms can be used when describing whether a compound is soluble or not. These terms are given in Table 1.1, and are based on the part of solvent needed to dissolve 1 part of the solute for example, testosterone is considered insoluble in water but soluble in alcohol, ether, or other organic solvents. Fortunately, when injected into the body, insoluble testosterone is diluted, and the larger volume of body water permits testosterone to go into the solution.
| Term | Parts of solvent required per part of solute |
| Very soluble | Less than 1 part |
| Freely soluble | 1 – 10 |
| Soluble | 10–30 |
| Sparingly soluble | 30–100 |
| Slightly soluble | 100 – 1000 |
| Very insoluble | 1000 – 10,000 |
| Insoluble | More than 10,000 |
Saturated Solution
A solution in which the dissolved solute is in equilibrium with the undissolved solute or solid phase is known as a saturated solution. It is when no more of the solid will dissolve into the solution. When we add a solute to a solvent,, a point is reached where no more solute dissolves under specified conditions. The solution is saturated. The concentration of the solute in a saturated solution is the solubility of the solute in that solvent at that temperature. Saturation of solution can also be defined as the point where the solution is in equilibrium with undissolved solute. In a saturated solution containing an undissolved solid solute, the rate at which the molecules or ions leave the solid surface is equal to the rate at which the solvated molecules return to the solid.

In Fig. 1.1, KSOL is the rate constant at which the solid is solvated, and KPPT is the rate constant at which the solvated molecule is returned to the solid. The solubility of a substance is the ratio of these rate constants at equilibrium in a given solution. At equilibrium, the rate at which a solute precipitates out of the solution is equal to the rate at which the solute goes into the solution.
Unsaturated Solution
An unsaturated solution is a solution containing the dissolved solute at a concentration lower than that of a saturated solution. If less solute is added to the solvent, then the solution is said to be unsaturated. Most pharmaceutical solutions are considered to be unsaturated.
Supersaturated Solution:
A solution that contains a higher concentration of solute than the saturated solution is known as a supersaturated solution. It requires an increase in temperature to make it possible to dissolve more into the solvent than is required to produce a saturated solution. This yields a supersaturated solution. These solutions can be prepared by heating the saturated solutions at higher temperatures. The solute is dissolved in the solvent at a high temperature, and then the solution is slowly cooled. Such a solution is unstable, and the addition of a small amount of solute causes all of the excess dissolved solute to crystallize out of the solution.
A saturated potassium chloride solution at 10 degrees Celsius will have 31 grams of this substance dissolved in 100 grams of water. If there are 40 grams of potassium chloride in the container, then there will be 9 grams of undissolved potassium chloride remaining in the solution. Raising the temperature of the mixture to 30 degrees Celsius will increase the amount of dissolved potassium chloride to 37 grams, and there will be only 3 grams of solids undissolved. The entire 40 grams can be dissolved if the temperature is raised above 40 degrees Celsius. Cooling the hot solution (40 degrees Celsius) will reverse the process. When the temperature decreases to 20 degrees Celsius, the solubility will eventually decrease to 34 grams. There is a time delay before the extra 6 grams of dissolved potassium chloride crystallize. This solution is “supersaturated” and is a temporary condition. The “extra” solute will come out of the solution when the randomly moving solute particles can form the crystal pattern of the solid. A “seed” crystal is sometimes needed to provide the surface for solute particles to crystallize on and establish equilibrium.
Concentration Units:
A wide range of units is commonly used to express solution concentration, and confusion often arises in the inter-conversion of one set of units to another. Wherever possible throughout this book, we have used the SI system of units. Although this is the currently recommended system of units in Great Britain, other more traditional systems are still widely used, and these are also used in the latter sections.
Also read: Suppositories, preparation of suppositories