Freeze-drying or freeze dryer, also called lyophilization, is a process in which water is frozen, followed by its removal from the sample, initially by sublimation followed by desorption. The equipment used to dry solutions or suspensions at or below freezing points of liquids is called a freeze dryer or lyophilizer. This drying process is utilized in the manufacture of pharmaceuticals and biologicals that are thermolabile or otherwise unstable in aqueous solutions for prolonged storage periods, but that is stable in the dry state.
Table of Contents
Principle of freeze dryer
The main principle involved in freeze-drying is a phenomenon called sublimation, where water passes directly from a solid-state (ice) to the vapor state without passing through the liquid state. Sublimation of water can take place at pressures and temperatures below the triple point of water (4.579 mm of Hg and 0.0099 ºC). The material to be dried is first frozen and then subjected under a high vacuum to heat (by conduction or radiation or by both) so that frozen liquid sublime, leaving only non-volatile solid, dried components of the original liquid. The concentration gradient of water vapour between the drying front and condenser is the driving force for the removal of water during freeze drying.
Construction of freeze dryer
Generally, there are three types of freeze dryers. There are manifold freeze-dryer, the rotary freeze dryer, and the tray-style freeze-dryer. The components common to all of them are a vacuum pump to reduce the ambient gas pressure in a vessel containing the substance to be dried and a condenser to remove the moisture by condensation on a surface cooled to − 20 to − 80°C. The manifold, rotary, and tray-type freeze-dryers differ in the method by which the dried substance is interfaced with a condenser.
- In manifold freeze-dryers a short usually circular tube is used to connect multiple containers with the dried product to a condenser.
- The rotary freeze-dryers have a single large reservoir for the dried substance. Rotary freeze-dryers are usually used for drying pellets, cubes and other pourable substances. The rotary dryers have a cylindrical reservoir that is rotated during drying to achieve a more uniform drying throughout the substance.
- The tray freeze-dryers also have a single large reservoir for the dried substance. They usually have rectangular reservoir with shelves on which products, such as pharmaceutical solutions and tissue extracts, can be placed in trays, vials and other containers.
A freeze dryer consists of a vacuum chamber wherein products to be dried are kept on shelves and capable of cooling and heating containers and their contents. A vacuum pump, a refrigeration unit, and associated controls are connected to the vacuum chamber.
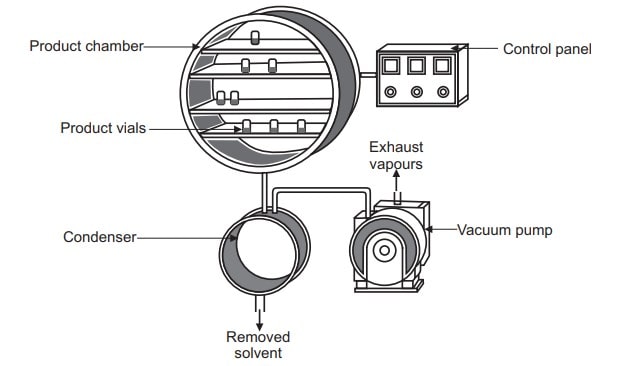
Working of freeze dryer
Traditional freeze drying is a complex process that requires a careful balancing of product, equipment, and processing techniques. In this process water is removed from a product after it is frozen and placed under a vacuum, allowing the ice to change directly from solid to vapour without passing through a liquid phase. It is performed at temperature and pressure conditions below the triple point of liquid, to enable sublimation of frozen material. The entire process is performed at low temperature and pressure. Steps involved in lyophilization start from sample preparation followed by freezing, primary drying and secondary drying, to obtain the final dried product. The concentration gradient of water vapour between the drying front and condenser is the driving force for removal of water during lyophilization. The vapour pressure of water increases with an increase in temperature during the primary drying. Therefore, primary drying temperature should be kept as high as possible, but below the critical process temperature, to avoid a loss of cake structure.
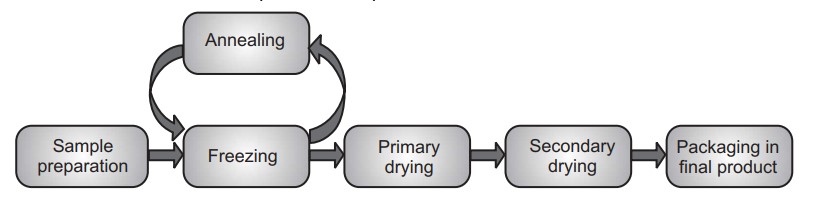
There are four important stages in the complete freeze drying process namely pretreatment, freezing, primary drying, and secondary drying.
(i) Pretreatment: In this stage product is treated for freeze concentration, solution-phase concentration, preserve product appearance, stabilize reactive products, increase surface area, and decrease high vapor pressure solvents concentration prior to freezing. In many instances, the decision to pre-treat a product is based on theoretical knowledge of freeze-drying and its requirements or is demanded by cycle time or product quality considerations.
(ii) Freezing: During the freezing stage, the liquid sample is cooled down to − 40 to − 60 °C until pure crystalline ice forms from part of the liquid and the remainder of the sample is freeze-concentrated into a glassy state where the viscosity is too high to allow further crystallization.
(iii) Primary drying: In primary drying the ice formed during the freezing is removed by sublimation under vacuum at low temperatures, leaving a highly porous structure in the remaining amorphous solute that is typically 10% water. This step is carried out at pressures of 10−4 to 10−5 atmospheres, and a product temperature of – 45 to – 20 °C. The sublimation during primary drying is the result of coupled heat- and mass-transfer processes.
(iv) Secondary drying: This is last step wherein most of the remaining water is desorbed from the glass as the temperature of the sample is gradually increased upto 10 – 15 °C while maintaining low pressures. Ideally, the final product is a dry, easily reconstituted cake with a high surface area and low moisture content (below 5% w/w).
Advantages
(i) Oxidizable substances are well protected under vacuum conditions.
(ii) Long drying period owing to 95%-99.5% water removal.
(iii) Loaded quantities are accurate and have content uniformity.
(iv) Little contamination owing to the aseptic process.
(v) Minimal loss in volatile chemicals and heat-sensitive nutrient and fragrant components.
(vi) Minimal changes in the properties because microbe growth and enzyme effect can not be exerted under low temperature.
(vii) Transportation and storage of thermostable products is possible under normal temperature.
(viii) Rapid reconstitution time, usually less than 10 sec.
(ix) Constituents of the dried material remain homogeneously dispersed.
(x) Sterility of product can be achieved and maintained.
Disadvantages
(i) Removing volatile compounds may require a high vacuum.
(ii) Most expensive unit operation.
(iii) Stability problems such as low-temperature stress are associated with individual drugs.
(iv) There are some issues associated with sterilization and sterility assurance of the dryer chamber and aseptic loading of vials into the chamber.
Applications of freeze dryer
(i) Pharmaceutical companies often use freeze-drying to increase the shelf life of products, such as vaccines and other injectables.
(ii) By removing the water from the material and sealing the material in a vial under a vacuum, the material can be easily stored, shipped, and later reconstituted to its original form for injection.
(iii) Freeze-drying is used to preserve biologicals and make it very lightweight.
(iv) It is used to preserve blood products in freeze-dried form.
(v) It is used in the chemical synthesis where products are often freeze-dried to make them more stable, or easier to dissolve in water for subsequent use.
(vi) As freeze-drying can effectively remove solvents that can be used in bio-separations as a late-stage of the purification procedure.
(vii) In addition, it is capable of concentrating substances with low molecular weights that are too small to be removed by a filtration membrane.
Batch and continuous type fluidized bed dryer