Table of Contents
Primary Lymphoid Organs
Lymphocytes must undergo a maturation-and-differentiation
process before they become fully immunocompetent. The maturation
of T and B cells takes place in different anatomic sites.
B cells undergo maturation in bone marrow or intestinal lymphoid
tissue. The thymus is responsible for T cell maturation.
Thymus
The thymus gland is found directly behind the sternum in the chest cavity.
The thymus is a multi-lobed enclosed structure. Each lobe has an exterior section known as the cortex and an interior portion known as the medulla. the medulla is a phrase used to describe a part of the brain that is. Within a haphazardly organized environment, Dendritic epithelial cells have a three-dimensional structure. Hassall’s corpuscles, thymocytes, nurse cells, and cells. ThisThe stroma, or cortical epithelial framework, gives a distinct feature. T cell maturation takes place in a specific environment. Lymphocytes from the bone marrow Thymocytes are the cells that enter the thymus. These cells will eventually die. T cells that have reached maturity.
At birth, the thymus is one of the largest organs in the
body with a weight of 25 to 30 g, and it continues to grow and
expand until puberty. During puberty, sex hormones cause the
thymus to atrophy (involute), and its normal architecture is
replaced by fat. After puberty, the hormones secreted by the
epithelium are important in the maintenance of activated lymphocytes.
By 30 years of age, only vestiges of the thymic epithelium
remain.
Maturation of T Cells in the Thymus
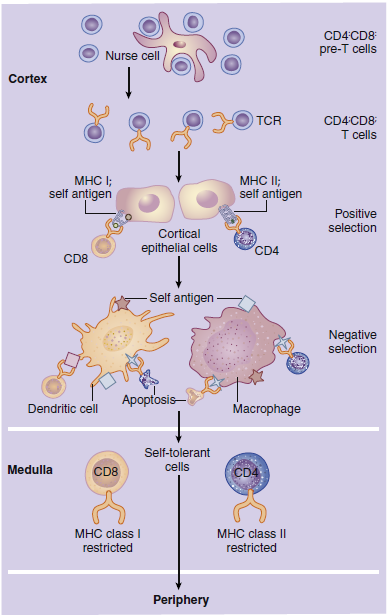
In the cortex, immature T cells begin an initial round of proliferation
during which the cortex becomes densely packed with
lymphocytes. Cortical epithelial cells called nurse cells sustain
proliferation by secreting interleukin 7 (IL-7). As they navigate
the stromal network from the cortex to the medulla, thymocytes
undergo a maturation-and-differentiation process. During maturation, a genetic rearrangement of TCRs
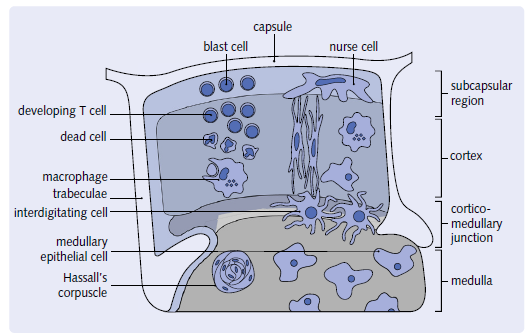
thymocytes, which are lymphocytes originating from the bone marrow. Their maturation begins in the subcapsular
region and ends in the medulla.
ensures that at least one lymphocyte that recognizes each of the
1013 possible antigens (protein or carbohydrate molecules recognized
as foreign by the host) is present. Unfortunately, some
of the maturing T cells now recognize antigens expressed by host
cells as well. These auto-reactive cells must be destroyed before
they can attack host tissue. Auto-reactive cells are removed by a
two-step—positive and negative—selection process.
In positive selection, thymocytes react with major histocompatibility
complex (MHC) molecules expressed on cortical epithelial
cells. MHC molecules are present on all nucleated cells
and serve two functions: (1) They are markers of “self ” or host
tissue, and (2) they present antigens to lymphocytes. The survival
or death of thymocytes is dependent on their affinity for
binding to the MHC molecules. Thymocytes that do not interact
with MHC molecules undergo apoptosis and are phagocytosed
in Hassell’s corpuscles. Cells binding to the MHC markers
with high affinity are considered auto-reactive; they undergo
apoptosis or are prevented from maturation. Cells binding to
the self-markers with low affinity are considered nonthreatening
to the host, and they move deeper into the cortex.
Negative selection removes thymocytes that have autoreactivity
to the self-antigens that are unique to tissue such
as the thyroid, muscle, intestinal, or neural tissue. In negative
selection, tissue-specific antigens are presented in the context
of MHC molecules expressed on dendritic cells, macrophages,
and thymic epithelial cells. Again, thymocytes that bind with
high affinity are considered auto-reactive, and they undergo
apoptosis. T cells with little or no affinity for the self-antigens
are allowed to enter the peripheral blood.
Hormones and T Cell Maturation
T cell maturation is under the control of the hormones secreted
by the thymic epithelium. These hormones include thymosin
α1, thymopoietin, thymopentin, thymosin β4, and thymulin.
On hormonal stimulation, most thymocytes express a
CD8+ marker but quickly transition into dual positive (CD4+,
CD8+) cells. Over 80% of the total cells in the adult thymic
cortex are dual positive. After 12 to 19 days of maturation,
only 20% of the original thymocyte population remains in
the thymus. Mature CD4+, CD8-, and TCR+ cells (12%–15%
of the total population) are released into peripheral blood as
CD4 T helper/amplifier cells. CD4-, CD8+, and TCR+
cells (3% of total thymocytes) are also released into peripheral
blood in high numbers to become CD8 cytotoxic cells.
A small percentage of cells (2%) that have TCRs but no surface
markers (CD4-, CD8-, TCR+) also are seeded into the peripheral
blood. These cells represent a small population of T cells that
have escaped the selection process.
Bone Marrow and Peyer’s Patches
The B cell maturation process in humans is still being debated.
Some data suggest that intestinal Peyer’s patches and other
gut-associated lymphoid tissue play critical roles in the differentiation
and maturation of B cells. Other evidence indicates
that bone marrow is involved in B cell maturation. It is clear,
however, that B cells undergo a gene rearrangement similar to
that in T cells. Like T cells, B cells have a receptor (BCR) that
reacts with antigens. Therefore, gene rearrangements result in
B cells specific for each of the 1013 possible antigens.