Important biological molecules include carbohydrates, amino acids, proteins, lipids, nucleotides, and enzymes.
Table of Contents
Carbohydrates
The carbohydrates are sugars. Carbohydrates are composed of carbon, oxygen, and hydrogen, and the carbon atoms are normally arranged in a ring, with the oxygen and hydrogen atoms linked to them. The structures of glucose, fructose, and sucrose are shown in the figure below. When two sugars link up, the reaction occurring expels a molecule of water, and the resulting bond is called a glycosidic linkage.
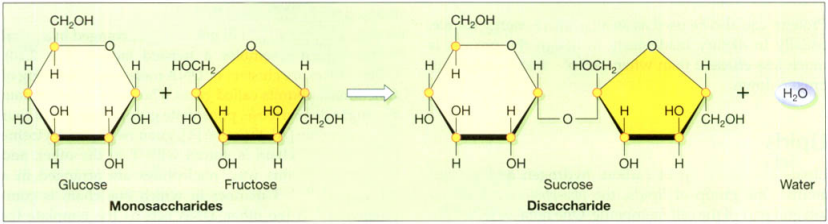
Simple sugars, like glucose, can exist as single units and are referred to as monosaccharides. Glucose is the main form in which sugar is used by cells, and blood levels are tightly controlled. Frequently, the monosaccharides are linked together, the resultant molecule ranging from two sugars or disaccharides, e.g. sucrose (table sugar), to long chains containing many thousands of sugars. Such complex carbohydrates are called polysaccharides, e.g. starch.
Glucose can be broken down (metabolized) in either the presence (aerobically) or the absence (anaerobically) of oxygen, but the process is much more efficient when O2 is used. During this process, energy, water, and carbon dioxide are released (p. 315). This family of molecules:
- Serves as a ready source of energy to fuel cellular activities
- Provides a form of energy storage, e.g. glycogen
- Forms an integral part of the structure of DNA and RNA
- Can act as receptors on the cell surface, allowing the cell to recognize other molecules and cells.
Amino acids and proteins-biological molecules
All amino acids also frequently carry sulfur. Amino acids are always composed of carbon, hydrogen, oxygen, and nitrogen. The primary building blocks of proteins in human biochemistry are 20 amino acids, although there are other amino acids that are employed in certain proteins or are only present in microbial products. There is a fundamentally similar structure among the amino acids utilized in the production of human proteins, consisting of an amino group (NH2), a carboxy group (COOH), and a hydrogen atom. A variable side chain distinguishes one amino acid from the next. The diagram below shows the fundamental structure as well as the three most prevalent amino acids. As in the formation of glycosidic linkages, when two amino acids join up the reaction expels a molecule of water and the resulting bond is called a peptide bond.
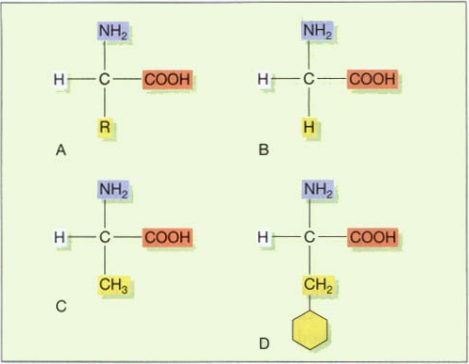
R = variable side chain. B. Glycine, the simplest amino acid.
C. Alanine. D. Phenylalanine.
Proteins are made from amino acids joined together, and are the main family of molecules from which the human body is built. Protein molecules vary enormously in size, shape, chemical constituents, and function. Many important groups of biologically active substances are proteins, e.g.:
- Carrier molecules, e.g. hemoglobin
- Enzymes
- Many hormones, e.g. insulin
- Antibodies
Proteins can also be used as an alternative energy source, usually in dietary inadequacy, although the process is much less efficient than when carbohydrates or fats are broken down.
Also read: Acids, alkalis and pH-in the body
Lipids-biological molecules
Lipids are made up of carbon, hydrogen, and oxygen atoms. One group of lipids, the phospholipids, forms an integral part of the cell membrane. One notable feature of lipid molecules is that they are strongly hydrophobic (water-hating) and therefore lipids do not mix with water. This is important in their function in the cell membrane.
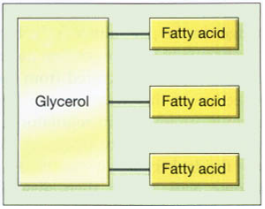
Certain vitamins (including E and K), a crucial class of hormones known as steroids, and fats are examples of other lipid kinds. A molecule of fat is made up of three fatty acids connected by glycerol molecules, as seen in the image below. In addition to being a source of energy, fats make it easy to retain extra calories. When fats are broken down, energy is released, but the process is less effective than when using carbs since the breakdown reaction needs more energy to occur. Their functions throughout the body include:
- Insulation
- Protection of body parts
- Energy storage
Nucleotides-biological molecules
Nucleic acids
These are the largest molecules in the body and are built from components called nucleotides, which consist of three subunits:
- a sugar unit
- a base
- one or more phosphate groups linked together
Deoxyribonucleic acid (DNA)
This is a double strand of nucleotides arranged in a spiral (helix) which resembles a twisted ladder figure shown below. Chromosomes are clusters of DNA molecules consisting of functional subunits called genes. The nucleotides contain the sugar deoxyribose, phosphate groups, and one of the four bases: adenine [A], thymine [T], guanine [G], and cytosine [C]. A in one chain is paired with T in the other, and G with C. In this way, nucleotides are arranged in a precisely ordered manner in which one chain is complementary to the other. DNA acts as the template for protein synthesis and is stored safely in the nucleus.
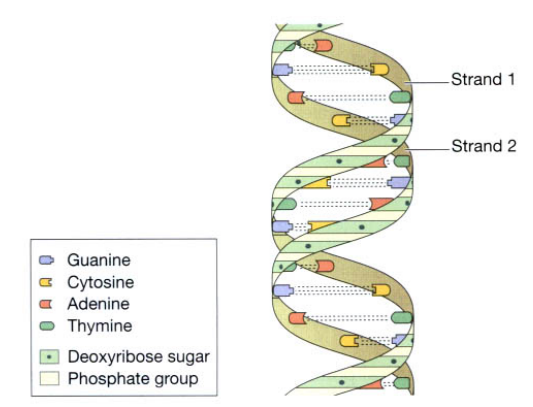
Ribonucleic acid (RNA)
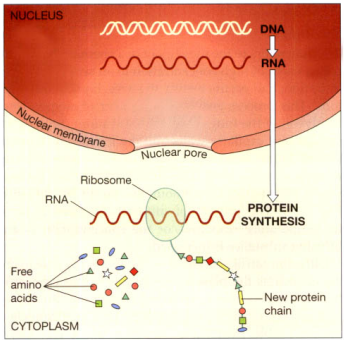
Instead of the deoxyribose present in DNA, this single-stranded chain of nucleotides includes the sugar ribose. Thymine is absent, and uracil is used in its place. It is created in the nucleus from a DNA template and sends instructions to the protein-synthesis machinery in the cell cytoplasm from the DNA, which is locked inside the nucleus and cannot be released.
Protein synthesis. When cells require new proteins, a single strand of RNA is made using DNA as the template; the RNA leaves the nucleus. RNA acts as the messenger, which carries the instructions for the assembly of the new protein to tiny structures in the cytoplasm called ribosomes. Ribosomes read the message and, following the instructions, assemble the new protein from amino acids in the cell cytoplasm. New chains of protein are often large molecules that coil up in a particular way to maintain the stability of the molecule.
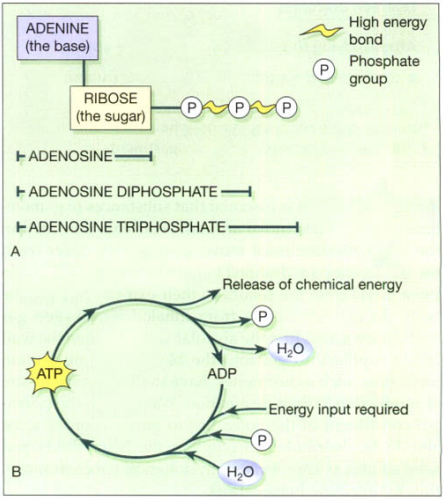
Adenosine triphosphate (ATP)
ATP is a nucleotide that contains ribose (the sugar unit), adenine (the base), and three phosphate groups attached to the ribose, shown in figure below. It is sometimes known as the energy currency of the body, which implies that the body has to ‘earn’ (synthesize) it before it can ‘spend’ it. Many of the body’s huge number of reactions release energy, e.g. the breakdown of sugars in the presence of O2. The body captures the energy released by these reactions, using it to make ATP from adenosine diphosphate (ADP). When the body needs chemical energy to fuel cellular activities, ATP releases its stored energy, water, and a phosphate group through the splitting of a high-energy phosphate bond, and reverts to ADP. The body needs chemical energy to:
- Drive synthetic reactions (i.e. building biological molecules)
- Fuel movement
- Transport substances across membranes.
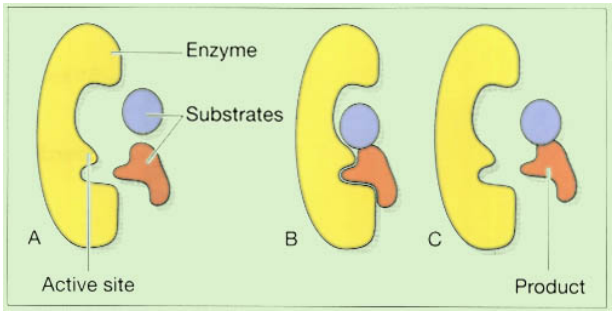
Enzymes
Many chemical processes in the body may be mimicked in a test tube. Surprisingly, the pace at which the reactions proceed frequently drastically decreases to the point where chemical activity effectively stops. The body’s cells have created an apparent answer to this issue by providing a vast assortment of enzymes. Proteins called enzymes operate as catalysts for biological reactions; they speed up the reaction without altering it, allowing them to be utilized repeatedly. Enzymes are very selective and often only catalyze one particular process. The molecules that start the reaction are referred to as the substrate, and they attach to the enzyme’s active site, which is a highly particular location. The reaction continues while the substrate(s) is bound to the active site, and when the reaction is finished, the product(s) of the reaction break away from the enzyme and the active site is ready for use again, figure shown in below. Enzymes can catalyze both synthesis and breakdown reactions, and their names (almost always!) end in ~ase.